First Report of a Providencia stuartii Strain Coproducing a Beta-Metalloenzyme NDM-1 Type and an Oxacillinase Type OXA-48
Karima Warda, Loubna Ait Said, Khalid Zerouli, Khalid Katfy, Kawtar Zahlane
Karima Warda1,2,*, Loubna Ait Said1, Khalid Zerouli3, Khalid Katfy3 and Kawtar Zahlane1,2
1Laboratory of Bacteriology, Ibn Tofail Hospital, Morocco
2Laboratory of Microbiology and Virology, University Cadi Ayyad, Morocco
3Laboratory of Microbiology, Ibn Rochd Hospital Rue Lahcen El Arjoun, Casablanca, Morocco
- *Corresponding Author:
- Karima Warda
Laboratory of Microbiology and Virology, University Cadi Ayyad, Morocco
Tel: +0212 670602083
E-mail: karimawarda@yahoo.fr/ ka.warda@ucam.ma
Abstract
Introduction: The emergence and dissemination of carbapenemase-producers enterobacterias and their worldwide spread represents a major issue for both clinical practice settings and public health strategies. Their current extensive spread worldwide is an important source of concern due to limited therapeutic options and raised risk of mortality.
Materials and methods: Pus samples obtained from burned patients admitted to the plastic surgery department were analyzed. They were seeded on ordinary and enriched culture media. Depending on the the antimicrobial susceptibility testing, research of metallo-ß-lactamase was done on isolates by EDTA synergy testing, Hodg test and multiplex PCR.
Results: The identification objectified the presence of multiresistant P. stuarti. Imipenem CMI was high (CMI>8 mg). The resistance has also interested other antibiotics with the exception of aztrinam, amikacin and ciprofloxacin. The EDTA synergy testing was positive (φ>5 mm). The Hodge test was negative for both strains. The PCR have demonstrated the presence of resistance genes (blaNDM-1, blaOXA-48).
Conclusion: This study demonstrated that multidrug resistant strains are present in our country. Regular monitoring and documentation of carbapenem resistant is crucial in developing strategies to control infection due to these bacteria.
Keywords
Providencia stuartii, Metallo-ß-enzyme, Nosocomial infection, Burn
Introduction
The genus Providencia includes gram-negative bacilli that are responsible for a wide range of human infections. Providencia stuartii (P. stuartii) is the most common species that can cause infections in humans. It is an opportunistic pathogen observed in patients with severe burns or those with long-term urinary catheters.[1] They represent an emerging problem because of the increasing prevalence of beta-lactam extended spectrum resistance (ESBL) and the recent emergence of carbapenem resistance.[2] We report the first cases of isolation of P. stuartii producing a beta-metalloenzyme (MBL) type NDM-1 and oxacillinase type OXA-48 in two burned patients hospitalized at the plastic surgery department in Ibn Tofail Hospital, university hospital center Marrakesh.
Materials and Methods
Sampling
In front of local and general signs of infection (feverish peaks and high CRP), skin samples were taken from two patients, a 34-year-old woman and a 25-year-old man, admitted to the plastic surgery department at the same time, victims of explosion of butane bottle, with burned cutaneous areas of 32% and 41% respectively. The skin samples were sent to the laboratory of the hospital Ibn Tofail for bacteriological analysis.
Inoculation
Samples received at the laboratory were inoculated in selective and non-selective media and incubated at 37°C for at least 24 hours.
Identification
P. stuartii isolates were identified by studying the biochemical characteristics using the API20E system (bioMérieux, Marcy l’Etoile, France). The strains were subsequently sent to Ibn Roch Casablanca UHC for genotypic study by real-time PCR.
Antimicrobial susceptibility testing
Susceptibility to various classes of antimicrobial agents was determined by the disc diffusion method in accordance with the recommendations of the French Society of Microbiology guidelines. The test medium was Mueller-Hinton. The Minimum inhibitory concentrations were determined by an automated method using the Becton Dickinson BD Phoenix. The antibiotics disks used in this study.
Phenotypic tests
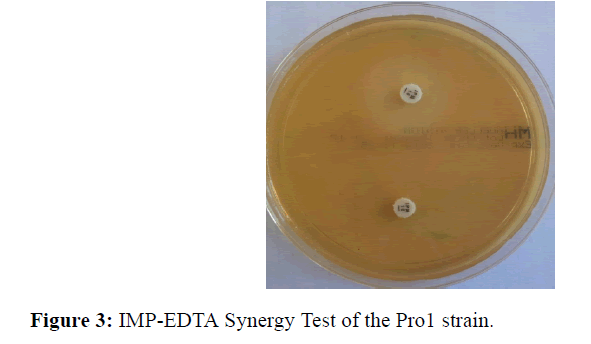
Synergy test EDTA: The EDTA synergy test was performed by placing the imipenem disks and an imipenem disk plus 10 μl of EDTA (Ethylene diamine tetraacetic acid) on Mueller agar. The test is positive when an increase in diameter greater than or equal to 5 mm between the zones of inhibition around the carbapenem disc and the combined disc IMP-EDTA is observed.
Hodge test: The modified Hodge test (MHT), which is recommended by CA-SFM as a confirmatory test for carbapenemase production was performed for each strain. A 0.5McFarland dilution of Escherichia coli in 5ml of broth was prepared. A 1/10 dilution was streaked on to a Meuller-Hinton agar plate. A 10 μg imipénème or ertapénème susceptibility disk was placed in the center of the test area. Test organism was streaked in a straight line from the edge of the disk to the edge of the plate. Two strains of Klebsiella pneumoniae were used as negative and positive controls. The plate was incubated overnight at 37°C for 24 h.
PCR (Polymerase chain reaction): The genotypic study was performed by multiplex PCR for the following carbapenemase genes blaNDM, blaVIM, blaKPC and blaOXA-48. The compositions of the primers for each gene studied are shown in Tables 1 and 2. Nucleic acid extraction was performed by kit marketed Mini Kit (QIAGEN). The realtime PCR was carried out by the Sybr Green technique on a CFX 96 real-time thermal cycler. The results of the PCR were interpreted by the quantization curve in relation to its threshold cycle (Ct) <25 cycles. For the confirmation of the results obtained two parameters have been added:
| Primers | Composition of the primers | Number of base pairs | Size of the gene |
|---|---|---|---|
| Sense primers (5’ à 3’) |
GCTTGATCGCCCTCGATT | 18 | 281 pb |
| Anti-sense primers (3’ à 5’) |
GATTTGCTCCGTGGCCGAAA | 20 |
Table 1: Composition of the primers of the OXA-48 gene.
| Gene | Composition of the primers | Number of base pairs | Tm | Size of the gene (pb) |
|---|---|---|---|---|
| KPC | 5’CATTCAAGGGCTTTCTTGCTGC3’ 3’ACGACGGCATAGTCATTTGC5’ |
22 20 |
60.93 58.99 |
538 |
| VIM | 5’ GATGGTGTTTGGTCGCATA3’ 3’CGAATGCGCAGCACCAG5’ |
19 17 |
55.61 59.54 |
390 |
| NDM | 5’GGTTTGGCGATCTGGTTTTC3’ 3’CGGAATGGCTCATCACGAT5’ |
20 20 |
60 62 |
621 |
Table 2: Oligonucleotide sequences of the primers used.
1. The threshold curve.
2. Migration of the amplifier on 1.5% agarose gel.
Results
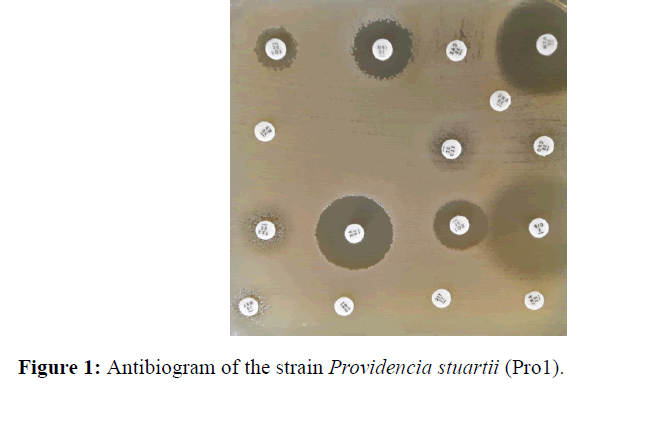
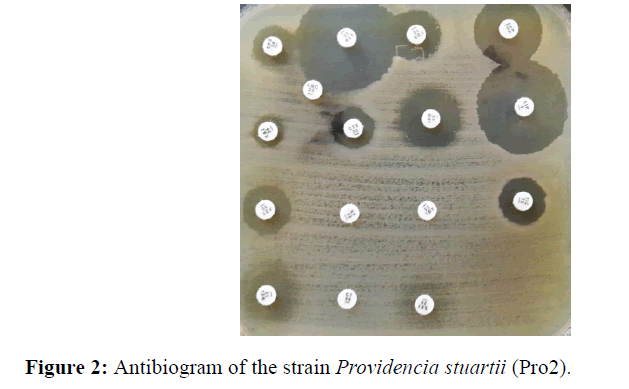
On both samples, a monomorphic shoot with multi resistant P. stuarti was observed (Figures 1 and 2). In front of this profile the search for a metallo-β-enzyme was carried out and showed that the growth of the bacterium was inhibited around the IMP-EDTA disk with a diameter of inhibition greater than 5 mm (Figure 3). The minimal inhibitory concentrations (MIC) at imipenem are higher than 8 mg/L. Antibiotic resistance also affected other families of antibiotics with relatively high MICs (Table 3), with the exception of amikacin and ciprofloxacin. The Hodge test was negative for both strains. The PCR result with the primers tested showed amplification with blaNDM-1 and blaOXA-48 (Table 4).
| Code | Antibiotics | Pro 1 | MIC | Pro 2 | MIC |
|---|---|---|---|---|---|
| AM | Ampicilline | R | >8 | R | >8 |
| AMX | Amoxicilline | R | R | ||
| TIC | Ticarcilline | R | >16 | R | >16 |
| PIP | Pipéracilline | R | R | ||
| AMC | Amoxicilline+acide clavulanique | R | >8/2 | R | >8/2 |
| Tic | Ticarcillin-Clavulanate | >16/2 | >16/2 | ||
| Piperacillin-Tazobactam | >16/4 | >16/4 | |||
| CF | Céfalotine | R | >32 | R | >32 |
| CXM | Céfuroxime | R | R | ||
| Céftriaxone | R | >4 | R | >4 | |
| CTX | Céfotaxime | R | R | ||
| CAZ | Céftazidime | R | >8 | R | >8 |
| FEP | Céfépime | R | >8 | R | >8 |
| FOX | Céfoxitine | R | >32 | R | >32 |
| Cefixime | R | >2 | >2 | ||
| ATM | Aztreonam | S | 2 | S | 2 |
| IMP | Imipénème | I | >8 | R | >8 |
| ERT | Ertapeneme | R | >1 | R | >1 |
| GEN | Gentamicine | R | >4 | R | >4 |
| TM | Tobramycine | S | S | ||
| AN | Amikacine | S | ≤ 4 | S | ≤ 4 |
| CIP | Ciprofloxacine | S | S | ||
| SXT | Triméthoprime/Sulfaméthoxazol | R | ≤ 1/19 S | R | ≤ 1/19 S |
| CS | Colistine | R | R | ||
| R=resistant, S=sensible, I=intermediate, Less than or equal (≤ ) | |||||
Table 3: Antibiotics tested and MIC.
| Strains tested | Pro 1 | Pro 2 |
|---|---|---|
| NDM-1 | POSITIVE | POSITIVE |
| VIM | NEGATIVE | NEGATIVE |
| OXA-48 | POSITIVE | POSITIVE |
| KPC | NEGATIVE | NEGATIVE |
Table 4: Result of the amplification by PCR in real time.
Discussion
The Providencia genus has five species, of which P. stuartii is the most frequently isolated from infections and the most resistant to antibiotics.[2] The high-level resistance to beta-lactamines in this species is mainly caused by the overexpression of its natural beta-lactamase type AmpC, but also by the acquisition of extended-spectrum betalactamases of the TEM-, SHV- or CTX-M type as well as than VEB-1 and PAR-1.[2]
The geographical distribution of resistant strains differs around the world; those that produce a KPC-type carbapenemase (class A) have been more frequently observed in the United States, Greece and Israel, OXA-48 (class D) is more common in the Mediterranean region including Morocco [3] while those producing beta-lactamases of type VIM and IMP class B were generally found in Asia and in the northern part of the Mediterranean basin.[4] Among these enzymes metallocarbapenemases are particularly worrying because they hydrolyze almost all classes of beta-lactams except aztreonam. Initially observed in strains of Pseudomonas aeruginosa and Acininitobactere spp., this resistance propagated in the Enterobacteriaceae family during the 1990s and 2000s.[4]
Discovered in 2008 in Sweden from an Indian patient, metallo-β-lactamase NDM-1 has already been identified on the majority of continents in 2010 and was linked in all cases with a stay in the subcontinent Indian.[5] Several epidemics or sporadic cases have been described in hospital or community settings.[6] This type of enzyme has been mainly isolated from K. pneumoniae, Enterobacteriaceae and recently from P. stuartii, and at various locations around the world.[7-10]
In our study, genotyping the two strains of P. stuartii isolated revealed the coexistence of the blaNDM-1 and blaOXA-48 genes. Other international studies have reported the coexistence of these two resistance genes and even several genes in both P. stuartii.[2,9,10] In Morocco the presence of beta-metalloenzyme-producing bacteria associated with OXA-48 has been reported in Klebsiella pneumoniae in Taza, Casablanca and Rabat. [3,11,12] To our knowledge, our study is the first to report the case of isolation of P. stuartii strains bearing bla-NDM-1 gene in Morocco, a region considered endemic for OXA-48. This is consistent with the fact suggesting the current spread of metallo-β-lactamase especially in clinical strains throughout the North African Countries.[3,11,13]
The level of carbapenem resistance is variable. The plasmids carrying the blaNDM-1 gene very often contain several other resistance genes, which ultimately leads to multiresistance or even total resistance of the strains.14 Strains isolated in this study showed resistance to all antibiotic families tested except aztreonam, tobramycin, amikacin and ciprofloxacin.
The hodge test in our study was negative whereas the genotyping of the strains shows the existence of beta-metallolo enzyme and oxacillinase genes. This result has also been reported by other studies, which means that this test is almost abandoned because of false negatives.[13]
The coexistence of these determinants of resistance in nosocomial strains is a threatening situation. The search for the clonal relationships between the P. stuartii isolates was not made in our study, but the phenotypic similarities of the strains suggest that it is the same strain that has spread from the first to the second patient. The rapid detection and the technical and geographical isolation of the two patients made it possible to limit the spread of this strain within the burn unit of the plastic surgery department and thus to prevent an epidemic.
The production of beta-metallo-lactamase in P. stuartii has been associated with an adverse evolution, most often fatal.[8,10] In our context, the evolution of both patients was favorable thanks to the combination of amikacin and ciprofloxacin in parallel with local care.
Conclusion
P. stuartii remains among the multiresistant strains prevalent in hospitals. In addition, the NDM-1 type metallo-β-lactamase producing strains represent an emerging threat in Morocco. The antimicrobial selection pressure could allow the silent propagation of the blaNDM-1 gene in Morocco. Thus, it seems necessary to start a serious and immediate management of this problem by implementing multidisciplinary strategies to optimize the detection and reduce the diffusion of strains producing MBL.
References
- Zavascki AP, Carvalhaes CG, da Silva GL, Tavares Soares SP. Outbreak of carbapenem-resistant Providencia stuartii in an intensivecare unit. Infect Control Hosp Epidemiol 2012; 33: 627-630.
- Galani L, Galani I, Souli M, Karaiskos I. Nosocomial dissemination of Providencia stuartii isolates producing extendedspectrum b-lactamases VEB-1 and SHV-5, metallo-b-lactamase VIM-1, and RNA methylase RmtB. JGAR 2013; 1: 115-116.
- Poirel L, Benouda A, Hays C, Nordmann P. Emergence of NDM-1-producing Klebsiella pneumoniae in Morocco. J Antimicrob Chemother 2011; 66: 2781-2783.
- Johnson AP, Woodford N. Global spread of antibiotic resistance: the example of New Delhi metallo-β-lactamase (NDM) mediated carbapenem resistance. J Med Microbiol 2013; 62: 499-513.
- Nordmann P, Poirel L, Walsh TR. The emerging NDM carbapénémases. Tren Microbiol 2011; 19: 588-595.
- Giske CG, Fröding I, Hasan CM, Turlej-Rogacka A. Diverse sequence types of Klebsiella pneumoniae contribute to the dissemination of blaNDM-1 in India, Sweden and the UK. Antimicrob Agents Chemother 2012; 56: 2735-2738.
- Arpin C, Thabet L, Yassine H, Messadi AA. Evolution of an incompatibility group IncA/C plasmid harbouring blaCMY-16 and qnrA6 genes and its transfer through three clones of Providencia stuartii during a two-year outbreak in a Tunisian burn unit. Antimicrobl Agents Chemother 2012; 56: 1342-1349.
- Bushnelle G, Mitrani-Gold F, Mundy LM. Emerging of New Delhi metallo-B-lactamase type1-producing Enterobacteriaceae and non-Enterobacteriaceae: global case detection and bacterial surveillance. Int J Infect Dis 2013; 17: 325-333.
- McGann P, Hang J, Clifford RJ, Yang Y. Complete sequence of a novel 178-kilobase plasmid carrying bla (NDM-1) in a Providencia stuartii strain isolated in Afghanistan. Antimicrob Agents Chemother 2012; 56: 1673-1679.
- Douka E, Perivolioti E, Kraniotaki E, Fountoulis K. Emergence of a pandrug-resistant VIM-1-producing Providencia stuartii clonal strain causing an outbreak in a Greek intensive care unit. Int J Antimicrob Agents 2015; 45: 533-536.
- Barguigua AD, El Otmani F, Talmi M, Zerouali K. Emergence of carbapenem resistant Enterobacteriaceae isolates in the Moroccan community, Diag Microbiol Infect Diseas 2012; 73: 290-291.
- Maroui I, Barguigua A, Aboulkacem A, Ouarrak K. First report of VIM-2 metallo-b-lactamases producing Pseudomonas aeruginosa isolates in Morocco. J Infect Chemother 2016; 22: 127-132.
- Castanheira M, Deshpande LM, Mathai D, Bell JM. Early Dissemination of NDM-1- and OXA-181-Producing Enterobacteriaceae in Indian Hospitals: Report from the SENTRY Antimicrobial Surveillance Program, 2006-2007. Antimicrob Agents and Chemother 201; 55: 1274-1278.
- Nordmann P, Naas T, Poirel L, Global spread of Carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis 2011; 17: 1791-1798.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences