The Development of Renal Injury is Accelerated in Obese Shrsp.Z-Leprfa/Izmdmcr Rats
Yasui N, Negishi H, Tsukuma R, Juman S, Miki T and Ikeda K
Yasui N*, Negishi H, Tsukuma R, Juman S, Miki T and Ikeda K
School of Pharmacy and Pharmaceutical Sciences, Mukogawa Women’s University, Nishinomiya, Japan
- *Corresponding Author:
- Naomi Yasui
School of Pharmacy and Pharmaceutical Sciences
Mukogawa Women’s University, 11-68, Nishinomiya 663-8179, Japan
Tel: 798-45-9993
Fax: 798-45-9993
E-mail: nyasui@mukogawa-u.ac.jp
Abstract
Metabolic syndrome is a risk factor for the development of diabetes and cardiovascular disease, and was recently linked to chronic kidney disease. Several metabolic disorders similar to metabolic syndrome patients occur in SHRSP.Z-Lepfa/ IzmDmcr rats (SPZF), which have a miss-sense mutation of leptin receptor gene from the genetic background of stroke-prone spontaneously hypertensive rats (SHRSP). These characters may affect renal function. We therefore investigated the development of renal injury in SPZF. SPZF at 12 weeks of age and age-matched their lean littermates (Lean) were used for physiological, blood and urine parameters. SPZF and Lean rats were sacrificed, and epididymal, mesenteric and retroperitoneal adipose tissues, kidney, and blood were sampled. Both SHRSP.ZF and Lean had hypertension because of the genetic background of SHRSP. SHRSP.ZF showed obesity, hyperglycemia, dislipidemia, and hyperinsulinemia as well as increased urinary excretion of urinary protein, albumin and neutrophil gelatinase-associated lipocalin in SPZF than in Lean. In histological analysis, the glomerular sclerosis score was significantly higher in SPZF than in Lean. Renal transforming growth factor beta 1 (TGF-β) and TGF-β receptor mRNA expressions in SPZF were significantly higher than in Lean. Serum monocyte chemoattractant protein-1 (MCP-1) was markedly higher (p<0.01) in SPZF than in Lean. Expressions of MCP-1 mRNA in SPZF retroperitoneal adipose tissue were significantly elevated compared with Lean. Kidney MCP-1 level in SPZF was also higher than in Lean. From these results, we concluded that SPZF at 12 weeks of age developed renal injury, the mechanism of which may be associated with TGF-β and MCP-1.
Keywords
SPZF; Renal injury; TGF-β; MCP-1
Introduction
Several epidemiological studies have linked metabolic syndrome to an increased risk for microalbuminuria, an early marker of kidney injury [1-3]. Recently, obesity has been reported to play important roles in the development of renal diseases directly and/or indirectly, and adipose tissue has attracted the attention of researchers [4].
Extensive experimental studies using animal models have greatly contributed to our understanding of metabolic syndrome and its complications in humans. Numerous experimental models have been used to study the pathogenesis, therapy, and prevention of obesity.
Zucker fatty (ZF) rats provide a model of obesity and insulin resistance as they carry a missense mutation in the extracellular domain of the leptin receptor [5] that causes hyperleptinemia, subsequently hyperphagia and obesity. ZF rats develop hyperinsulinemia, hypercholesterolemia and hypertriglyceridemia [6]. On the other hand, SHRSP, which are selectively bred from spontaneously hypertensive rats, are unique in that they develop severe hypertension quickly, above 250 mmHg. SHRSP develop hypertension-related disorders such as nephropathy, cardiac hypertrophy, and atherosclerosis similar to human essential hypertension, and die of stroke with 100% mortality [7].
We previously documented the establishment of a new animal model of metabolic syndrome, SPZF, by introducing a segment of the mutant leptin receptor gene from the ZF line heterozygous for the fa gene mutation into the genetic background of the SHRSP/Izm [8].
In the experimental rat model, the relationship is understood between obesity and renal injury. ZF is an autosomal-recessive model of obesity characterized by proteinuria and glomerular sclerosis, usually apparent after 14 weeks of age [9,10]. In the present study, we elucidated the change of renal function in SPZF at 12 weeks of age.
Methods
Animal model and experimental design
Five male SPZF and Lean at 11 weeks old, were obtained from the Disease Model Cooperative Research Association (Kyoto, Japan). All experiments in the present study conformed to Guidelines for the Care and Use of Laboratory Animals at Mukogawa Women’s University. All rats were housed under controlled conditions of constant temperature (22°C) and a light/dark cycle of 12 h with free access to food and water for one week. Each rat at 12 weeks old was weighed and placed in a metabolic cage for 24-h urine collection and diet intake. Rats were sacrificed, and epididymal, mesenteric and retroperitoneal adipose tissues, kidney, and blood were sampled.
Blood pressure measurement
Systolic blood pressure was measured by the tail-cuff method as previously described.11 The median of five successive measurements was used.
Blood sampling and analysis
Blood samples were obtained from the vena cava of rats. The blood samples were centrifuged, and serum and plasma were frozen at -70 degrees for subsequent measurement of blood parameters. Serum MCP-1, total cholesterol, triglyceride and free fatty acid (FFA) concentrations were measured and compared with those of SPZF and Lean (MCP-1, cholesterol E-test WAKO, Triglycerides E-test WAKO; Wako Pure Chemical Industries, Osaka, Japan).
Plasma leptin, insulin and glucose were measured in these samples (Rat Leptin, Rat Insulin, Morinaga Institute of Biological Science, Yokohama; Glucose CII-test WAKO, ELISA, Wako Pure Chemical Industries).
Urinary sampling and analysis
At 12 weeks old, each rat was placed in an individual metabolic cage to collect urine over 24 h, and urine samples were collected and frozen at -70 degrees for subsequent analysis. The urine samples were used to calculate urine volume, protein, albumin excretion, neutrophil gelatinase-associated lipocalin (NGAL, Rat ELISA Kit; Funakosi, Tokyo, Japan) and 8-hydroxy -2′-deoxyguanosine (8-OHdG) levels (8-OHdg Check, Japan Institute for the Control of Aging, Shizuoka, Japan).
Reverse transcription (RT) - polymerase chain reaction (PCR)
Total RNA was isolated from the kidney samples using RNeasy Mini kit (no. 74104, Qiagen, Tokyo, Japan), and first-strand complementary DNA was synthesized using SuperScript-III Reverse transcriptase (no. 18080-044, Invitrogen, Tokyo, Japan). The resulting complementary DNA was amplified with Quick Taq HS DyeMix. The amplified products were separated by electrophoresis on 1.5% agarose gel. The mRNA levels were corrected relative to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA. Imaging analysis of mRNA expression was performed using Scionimage (MD, U.S.A.). All reactions were performed in duplicate.
Histological analysis
Kidneys from rats were stained with periodic acid-Schiff (PAS) and examined by light microscopy in a blinded fashion. Glomerulosclerosis was semi-quantitatively evaluated according to criteria developed by Kanda et al [12]. Briefly, 50 glomeruli were randomly selected from each animal for morphometric analysis. The number of glomeruli exhibiting focal or global ischemic or proliferative damage was enumerated and expressed as a percentage of the total number of glomeruli examined.
Statistical analysis
Data are shown as the means ± SD. The statistical significance of differences in mean values was assessed using Student’s t test and the Mann-Whitney U test. A p-value of <0.05 was accepted as significant.
Results
Body weight, organ weight, food intake and blood pressure in SPZF and Lean
Body weight of SPZF, which showed hyperphagia, increased compared with that of Lean. The organ weights of the strains are shown in Table 1. Obesity is characterized by an increased mass of adipose tissue and increased size of adipocytes. We confirmed significantly increased epididymal, mesenteric, and retroperitoneal adipose tissue as visceral fat in SPZF. Both SPZF and Lean showed hypertension (Table 1).
| ÃÆãÃâââ¬Ãââ⬠| SPZF (n=5) | Lean (n=5) | F | t | df | p | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| BWT (g) | 377 | ± | 20.00 | 290 | ± | 11 | 4.1 | 8.63 | 5.9 | <0.01 |
| Kidneys (g) | 2.23 | ± | 0.18 | 2.23 | ± | 0.06 | 5.7 | -0.03 | 9.0 | 0.976 |
| Epi.fat (g) | 7.99 | ± | 0.58 | 3.28 | ± | 0.72 | 0.0 | 11.65 | 9.0 | <0.01 |
| Mes.fat (g) | 5.96 | ± | 0.69 | 2.35 | ± | 1.41 | 1.8 | 5.20 | 9.0 | <0.01 |
| Ret.fat (g) | 11.4 | ± | 0.94 | 2.88 | ± | 1.28 | 0.0 | 12.36 | 9.0 | <0.01 |
| Food intake (g) | 23.9 | ± | 0.12 | 30.0 | ± | 0.18 | 0.2 | 32.94 | 8.0 | <0.01 |
| SBP (mmHg) | 207 | ± | 30 | 222 | ± | 12 | 10.6 | -1.00 | 5.1 | 0.364 |
Table 1: Body weight, organ weight, food intake and blood pressure in SPZF and Lean.
Blood parameters in SPZF and Lean
Plasma insulin and glucose concentration were markedly augmented in SPZF rats compared with those in Lean. Similarly the levels of triglyceride and FFA were significantly higher in SPZF than in Lean. As shown in Table 2, plasma leptin was significantly higher in SPZF than Lean.
| SPZF (n=5) | Lean (n=5) | F | t | df | p | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| TG (mg/dl) | 373 | ± | 23 | 50 | ± | 6 | 9.9 | 13.77 | 4.5 | <0.01 |
| FFA (mEq/L) | 1.11 | ± | 0.13 | 0.49 | ± | 0.06 | 5.1 | 10.42 | 9.0 | <0.01 |
| Glucose (mg/dl) | 231 | ± | 28 | 160 | ± | 16 | 2.7 | 5.30 | 9.0 | <0.01 |
| Insulin (ng/ml) | 34 | ± | 26 | 0.7 | ± | 0.2 | 37.4 | -2.93 | 4.0 | <0.05 |
| Leptin (ng/ml) | 50 | ± | 6 | 0.7 | ± | 0.2 | 22.9 | 17.78 | 4.0 | <0.01 |
Table 2: Metabolic parameters in SPZF and Lean.
Urinary renal function and renal histology in SPZF and Lean
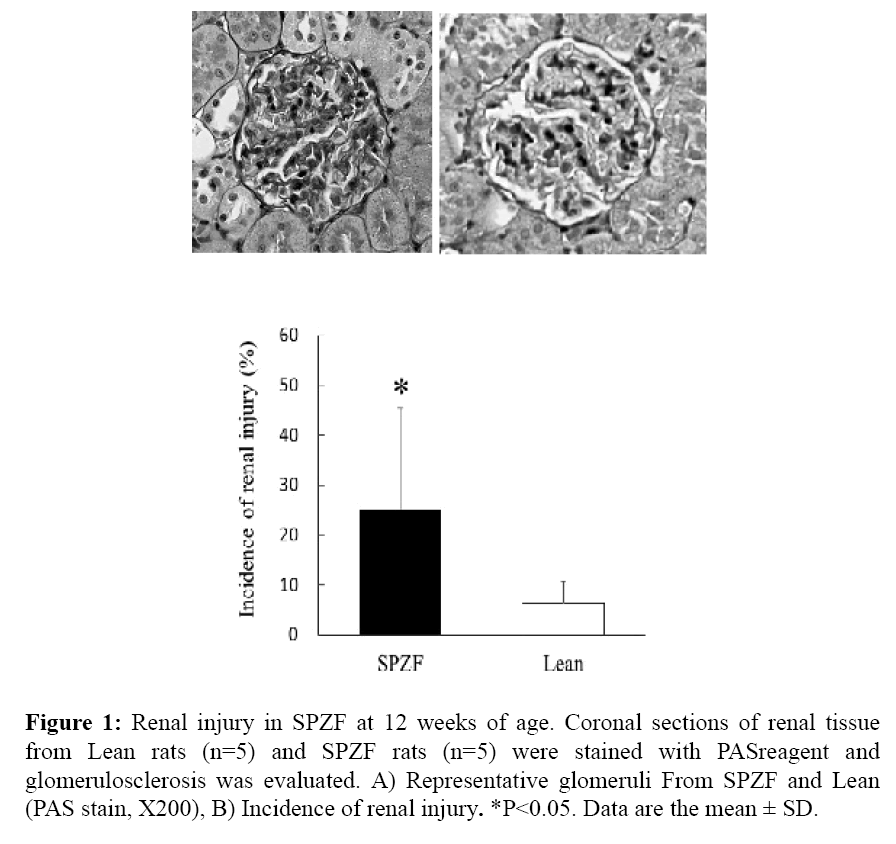
Urinary protein and albumin in SPZF were higher than in Lean. As NGAL has been considered as a real-time indicator of active kidney damage, urinary NGAL was also significantly higher in SPZF than in Lean. Furthermore, urinary 8-OHdG was measured as a marker of oxidative stress. The total amounts of urinary 8-OHdG excretion were greater in SPZF than in Lean rats (Table 3). In histological analysis, morphological change such as increased mesangial matrix were observed (Figure 1A), and the glomerular sclerosis score was significantly higher in SPZF than in Lean (Figure 1B).
| SPZF (n=5) | Lean (n=5) | F | t | df | p | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| uTP (mg/day) | 30.2 | ± | 12.5 | 18.5 | ± | 1.6 | 3.4 | 2.29 | 9.0 | <0.05 |
| Alb(mg/day) | 1042 | ± | 742 | 84 | ± | 36 | 12.0 | 2.88 | 4.0 | <0.05 |
| NGAL(U/day) | 9390 | ± | 2827 | 5843 | ± | 1055 | 4.0 | 2.87 | 9.0 | <0.05 |
| 8OH-dG(ng/day) | 257 | ± | 145 | 77 | ± | 17 | 33.2 | 2.76 | 4.1 | <0.05 |
| Urine Volume (g) | 33.8 | ± | 13.4 | 18.0 | ± | 7.3 | 4.9 | 2.49 | 9.0 | <0.05 |
Table 3: Urinary renal function parameters in SPZF and Lean.
Figure 1: Renal injury in SPZF at 12 weeks of age. Coronal sections of renal tissue from Lean rats (n=5) and SPZF rats (n=5) were stained with PASreagent and glomerulosclerosis was evaluated. A) Representative glomeruli From SPZF and Lean (PAS stain, X200), B) Incidence of renal injury. *P<0.05. Data are the mean ± SD.
Expression of TGF-β and TGF-β receptor mRNA in SPZF and Lean
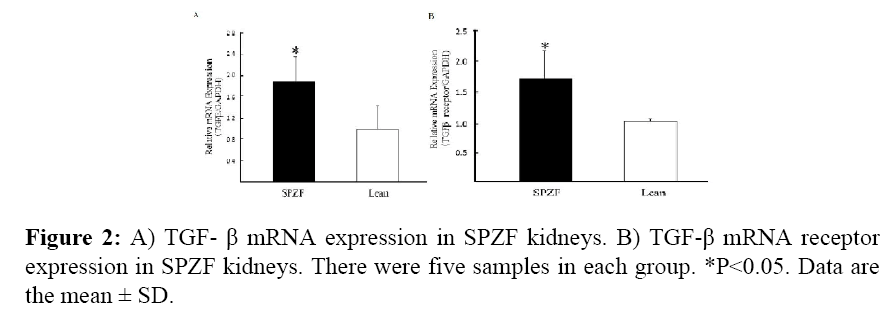
In renal gene expression, SPZF was associated with higher renal mRNA expression of TGF-β and TGF-β receptor than in Lean (Figures 2A and 2B).
Expression of MCP-1 in SPZF and Lean
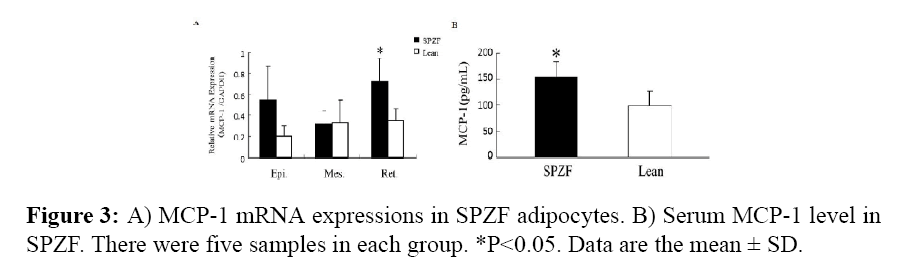
In SPZF, epididymal, retroperitoneal and mesenteric adipose tissues were markedly higher in mass compared than those in Lean. Increased MCP-1 mRNA expression in SPZF adipose tissues was particularly higher. MCP-1 mRNA expression (p<0.05) in retroperitoneal adipose tissue (Figure 3A). The serum level of MCP-1 was significantly higher in SPZF (Figure 3B).
Discussion
We observed increased urinary albumin, NGAL, which are diagnostic kidney injury markers, and morphological change of the kidneys in 12-week-old SPZF. These results suggest that kidney injury in SPZF at the age of 12 weeks is more severe than in agematched Lean.
SPZF developed to introduce a segment of the mutant leptin receptor gene from the ZF line heterozygous for the fa gene mutation into the genetic background of the SHRSP/ Izm. Lean has the fa (+/+) gene into the genetic background of the SHRSP/Izm.
Urinary protein excretion of 12 week old ZF was significantly decreased compared with same age SPZF (data not shown).
Hypertension accelerates the progression toward end-stage renal failure. Both SPZF and Lean rats showed severe hypertension, and their hypertension was not different between the two strains. Although hypertension plays a role in the development of renal failure, the difference in renal failure between SPZF and Lean may have relation to another factor.
SPZF showed an increased appetite by a missense mutation of the leptin receptor gene as shown in Table 1. In reference to increased diet intake, Baylis et al. reported that the total amount of food ingested by rats during their life affect the extent of renal damage [13]. Hyperphagia in SPZF may affect renal injury.
Several other causes increased kidney injury in SPZF at the age of 12 weeks, one of which is the leptin level. Our data showed increased plasma leptin concentration in SPZF, which derived ZF. Takaya et al. documented that the leptin receptor has a missense mutation, OB-R (5), and OB-R mRNA was present in the brain from ZF (2). Leptin has direct effects on renal pathophysiological characteristics [14]. In glomerular endothelial cells, leptin stimulates cellular proliferation, transforming growth factor-beta (TGF-β) synthesis [14,15]. Our results confirmed that TGF-β expression in the kidney was increased in SPZF. We further observed that hyperlipidemia and abnormalities in lipid metabolism frequently accompany renal disease and may be important in the pathogenesis of progressive renal injury in rats [16]. There is evidence linking hyperlipidemia to progressive renal injury in various experimental models, and it is believed to be mediated via oxidized LDL by a mechanism involving the expression of TGF-β [17]. We also described dyslipidemia and the increased expression of TGF-β in the kidneys. TGF-β is a protein that in most cells controls proliferation and differentiation. TGF-β stimulates collagen biosynthesis, which may lead to tissue fibrosis. Therefore, increased leptin and hyperlipidemia induced TGF-β.
Several studies have suggested that insulin resistance affects renal injury [18,19]. Furthermore, Tateya et al. reported that an increase in the circulating concentration of MCP1 elicits systemic insulin resistance [20]. Our data showed that serum MCP-1 was increased in SPZF, and increased insulin resistance was observed.
Recent studies have shown that fat tissue is not a simple energy storage organ, but was important endocrine and immune functions. MCP-1 is one of the key chemokines that regulate the migration and infiltration of monocytes/macrophages. Although MCP-1 expression was not increased in SHR and stroke-prone SHR kidneys, it was increased in angiotensin II-dependent models of hypertensive nephrosclerosis in SHR with streptozotocin-induced diabetes [21] and rats with two-kidney, one-clip hypertension [22]. ZF rats are the best known and most widely used animal model of genetic obesity, and it is reported that serum MCP-1 was higher in ZF rats suggesting an inflammatory state within visceral adipose tissue [23]. Recently, Tsuchiya et al. showed that exogenous administration of ANG II to rats increased MCP-1 mRNA expression in epididymal, subcutaneous, and mesenteric fat pads [24]. Our data showed increased mRNA MCP-1 expression in retroperitoneal adipose tissue in SPZF. This increased MCP-1 expression may increase serum and kidney concentrations, which are related to the development of kidney injury.
Previous our data suggest that 24 week old SPZF showed glomerular injury was significantly increased [25]. We newly observed 12-week-old SPZF showed increased renal injury, and easy to use examine the relationship between renal injury and obesity at young age of SPZF.
From these results, we concluded that 12-week-old SPZF developed renal injury spontaneously compared with Lean. Renal injury was caused by many factors, such as hyperphagia, dyslipidemia, high leptin concentration and elevated MCP-1.
Although the mechanisms by which obesity cause renal injury, in addition to hypertension, are still unclear, multiple factors have been proposed, including inflammation, mitochondrial dysfunction, oxidative stress, dyslipidemia, and lipotoxicity [26]. This model may be useful to investigate the mechanism and treatment of kidney injury with obesity.
Funding Information
The research in this study was supported in part by research grants from JSPS KAKENHI Grant Number 70399145.
References
- Mykkänen L, Zaccaro DJ, Wagenknecht LE, Robbins DC, Gabriel M, et al. (1998) Microalbuminuria is associated with insulin resistance in nondiabetic subjects: the insulin resistance atherosclerosis study. Diabetes 47: 793-800.
- Hoehner CM, Greenlund KJ, Rith-Najarian S, Casper ML, McClellan WM. (2002) Association of the insulin resistance syndrome and microalbuminuria among nondiabetic native Americans. The Inter-Tribal Heart Project. J Am SocNephro 13: 1626-1634.
- Palaniappan L, Carnethon M, Fortmann SP (2003) Association between microalbuminuria and the metabolic syndrome: NHANES III. Am J Hypertens 16: 952-958.
- Odermatt A (2011) The Western-style diet: a major risk factor for impaired kidney function and chronic kidney disease. Am J Physiol Renal Physiol 301: 919-931.
- Takaya K, Ogawa Y, Isse N, Okazaki T, Satoh N, et al. (1996) Molecular cloning of rat leptin receptor isoform complementary DNAs-Identification of a missesnse mutation in Zucker fatty (fa/fa) rats. BiochemBiophys Res Commun 225: 75-83.
- Ogawa Y, Masuzaki H, Isse N, Okazaki T, Mori K, et al. (1995) Molecular cloning of rat obese cDNA and augmented gene expression in genetically obese Zucker fatty (fa/fa) rats. J Clin Invest 96: 1647-1652.
- Yamori Y, Mori C, Nishio T, Ooshima A, Horie R, et al. (1979) Cardiac hypertrophy in early hypertension. Am J Cardiol 44: 964-969.
- Hiraoka-Yamamoto J, Nara Y, Yasui N, Onobayashi Y, Tsuchikura S, et al. (2004) Establishment of a new animal model of metabolic syndrome: SHRSP fatty (fa/fa) rats. ClinExpPharmacolPhysiol 31: 107-109.
- Coimbra TM, Janssen U, Gröne HJ, Ostendorf T, Kunter U, et al. (2000) Early events leading to renal injury in obese Zucker (fatty) rats with type II diabetes. Kidney Int 57: 167-182.
- Baynes J, Murray DB (2009) Cardiac and renal function are progressively impaired with aging in Zucker diabetic fatty type II diabetic rats. Oxid Med Cell Longev 2: 328-334.
- Nabika T, Nara Y, Ikeda K, Endo J, Yamori Y (1993) A new genetic locus cosegregating with blood pressure in F2 progeny obtained from stroke-prone spontaneously hypertensive rats and Wistar-Kyoto rats. J Hypertens 11: 13-18.
- Kanda T, Wakino S, Hayashi K, Homma K, Ozawa Y, et al. (2003) Effect of fasudil on Rho-kinase and nephropathy in subtotallynephrectomized spontaneously hypertensive rats. Kidney Int 64: 2009-2019.
- Baylis C, Corman B (1998) The aging kidney: insights from experimental studies. J Am SocNephrol 9: 699-709.
- Wolf G, Chen S, Han DC, Ziyadeh FN (2002) Leptin and renal disease. Am J Kidney Dis 39: 1-11.
- Wolf G, Hamann A, Han DC, Helmchen U, Thaiss F, et al. (1999) Leptin stimulates proliferation and TGF-beta expression in renal glomerular endothelial cells: potential role in glomerulosclerosis. Kidney Int. 56: 860-872.
- Kasiske BL, O'Donnell MP, Schmitz PG, Kim Y, Keane WF (1990) Renal injury of diet-induced hypercholesterolemia in rats. Kidney Int 37: 880-891.
- Ding G, van Goor H, Ricardo SD, Orlowski JM, Diamond JR (1997) Oxidized LDL stimulates the expression of TGF-beta and fibronectin in human glomerular epithelial cells. Kidney Int 51: 147-154.
- Fujiwara K, Hayashi K, Ozawa Y, Tokuyama H, Nakamura A, et al. (2000) Renal protective effect of troglitazone in Wistar fatty rats. Metabolism 49: 1361-1364.
- Kasiske BL, O'Donnell MP, Keane WF (1992) The Zucker rat model of obesity, insulin resistance, hyperlipidemia, and renal injury. Hypertension 19: I110-115.
- Tateya S, Tamori Y, Kawaguchi T, Kanda H, Kasuga M. (2010) An increase in the circulating concentration of monocyte chemoattractant protein-1 elicits systemic insulin resistance irrespective of adipose tissue inflammation in mice. Endocrinology 151: 971-979.
- Ota T, Takamura T, Ando H, Nohara E, Yamashita H, et al. (2003) Preventive effect of cerivastatin on diabetic nephropathy through suppression of glomerular macrophage recruitment in a rat model. Diabetologia 46: 843-851.
- Hilgers KF, Hartner A, Porst M, Mai M, Wittmann M, et al. (2000) Monocyte chemoattractant protein-1 and macrophage infiltration in hypertensive kidney injury. Kidney Int 58: 2408-2419.
- Miranville A, Herling AW, Biemer-Daub G, Voss MD. (2012) Differential adipose tissue inflammatory state in obese nondiabeticZucker fatty rats compared to obese diabetic zucker diabetic fatty rats. HormMetab Res 44: 273-278.
- Tsuchiya K, Yoshimoto T, Hirono Y, Tateno T, Sugiyama T, Hirata Y (2006) Angiotensin II induces monocyte chemoattractant protein-1 expression via a nuclear factor- B-dependent pathway in rat preadipocytes. Am J PhysiolEndocrinolMetab291: E771–E778.
- Ueno T, Takagi H, Fukuda N, Takahashi A, Yao EH, et al. (2008) Cardiovascular remodeling and metabolic abnormalities in SHRSP.Z-Lepr(fa)/IzmDmcr rats as a new model of metabolic syndrome. Hypertens Res 31:1021-1031.
- Hall JE, Henegar JR, Dwyer TM, Liu J, Da Silva AA, et al. (2004) Is obesity a major cause of chronic kidney disease? AdvRen Replace Ther 11: 41-54.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences