Practical MR Imaging of Anal Fistula Disease: How We Do It
Piloni Vittorio, Chiavarini Marco, Fabbroni Luigi, Possanzini Marco, Bellido Aleman Maritza, Sartini Marika
Piloni Vittorio*, Chiavarini Marco, Fabbroni Luigi, Possanzini Marco, Bellido Aleman Maritza and Sartini Marika
Radiology Department, Villa Igea Clinic, Via Maggini 200, Ancona, Italy
- *Corresponding Author:
- Piloni Vittorio
Radiology Department, Villa Igea Clinic
Via Maggini 200, Ancona, Italy
Tel: 0039719948000
E-mail: vittorio.piloni@libero.it
Abstract
Aim: A standard MRI technique of the pelvis is described which is well tolerated by patients and helps clinicians in surgical planning. Materials and methods: The clinical and imaging data of sixteen consecutive symptomatic men (mean age 45.5 ± 2 yrs, range 24-73 yrs) and fourteen women (mean age 40.6 ± 1.8 yrs, range 19-57 yrs) with known or suspected ano-perianal sepsis, who underwent MRI study between July 2015 and July 2016 were reviewed. The examinations were performed on a 1.5 T horizontal scanner using an external coil, T2–W and STIR pulse sequences in all three scan planes and an endoanal marker for evidence any intra o extra sphincteric collection, internal and external openings, distant extents and signs of disease activity despite apparent healing. The frequency, with which findings at MRI changed the preliminary clinical diagnosis and the subsequent surgical management from a simple fistula into that of complex fistula disease, was calculated. Results: The average time interval from the onset of symptoms to the request of MRI study was 13 ± 2 months (range 3-39 months) while only 5 out of 30 cases (16.6%) patients were evaluated with 3D endoanal-ultrasonography. Overall, in 27 out of 30 subjects (90%) a +440% increase in the rate of complex MR parameters was observed leading to need for reoperation and/or a more aggressive and extensive surgery. Conclusions: MRI is indicated as early as possible in the diagnostic work-up of anal fistula disease.
Keywords
Fistula-in-ano, Surgery, Magnetic resonance imaging, Preoperative MRI of anal sepsis
Introduction
Although the exact role of MR imaging in the pre and postoperative evaluation of ano-perianal sepsis has not been established yet [1], after a long period of skepticism, most prominent Italian colon proctologists involved in managing the disease have become more and more confident today in the use of this imaging modality. Two factors are thought to have prompted it, as follows: 1) a persistent high recurrence rate despite apparent successful surgery; and 2) a fear for loss of reputation and legal controversy triggered by patients in case of failed treatment. When compared to three dimensional (3- D) anal endosonography—the first line imaging modality in complex fistula disease— MRI is credited of better diagnostic accuracy, mainly due to its superior field of view and capability to characterize disease activity. Despite this, however, up to now MRI has received only limited attention by most surgeons, whose reluctance in relying on it may be explained with a sort of mistrust in the radiologists’ ability to put themselves in the surgeons’ place when producing the report. The aim of the present paper is two-fold, as follows: firstly, a standard easy-to-use MRI protocol is described, which hopefully will make its utilization and interpretation familiar also for non-radiologists; secondly (and most important), a clear take-home message will be sent to all proctologists, i.e. contrary to the general view [2] that most abscesses and fistulas do not require any imaging technique, the use of MRI is indicated in every case as early as possible and not in selected patients only , in order to detect occult abscess and secondary tracts.
Subjects and Methods
Patients’ reception
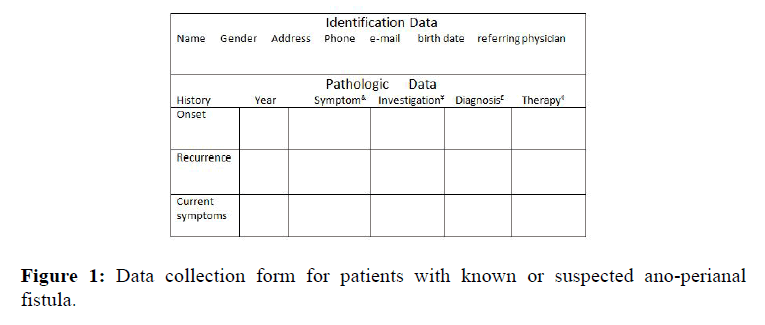
At their arrival in the radiology department, during the preliminary interview in a separate waiting room, patients are helped by the nurse staff (M.B.A, M.S) to fill in a form (Figure 1) which provides information on symptoms presentation since the onset of the disease up to the more recent state, including details on prior treatments, either medical or surgical. In particular, symptoms such as itching, swelling and pain of the ano-perianal region, with or without fever, discharge of pus material through the anal verge or through an external opening or passage of air and fecal material outside the vagina, are registered. During history taking, besides details on delivery and obstetric trauma (women), special attention is devoted to record data of either single or multiple surgical procedures due to recurrence of the disease, after apparent healing. In addition, patients are asked to exhibit their medical records and imaging series, if any, such as 2-D or 3-D anal endo sonography or magnetic resonance study of the pelvis. Furthermore, at the moment of the preliminary interview (average time 5 minutes), patients are asked to give written consent to the examination and cooperate actively to its success, after having been informed on duration (average time 24 ± 2 minutes) and need for insertion and maintenance of the catheter inside the anal canal during image acquisition without moving. The present report is based on data collected between July 2015 and July 2016 of sixteen consecutive symptomatic men (mean age 45.5 ± 2 yrs, range 24-73 yrs) and fourteen women (mean age 40.6 ± 1.8 yrs, range 19-57 yrs) with known or suspected ano-perianal sepsis. The diagnosis was usually suggested by the referring physician on the basis of medical history and clinical examination.
Imaging technique
After admittance in the diagnostic room, MR imaging studies (C.M, F.L, P.M.) for the assessment of perianal sepsis and fistulas are performed on a 1.5 T scanner (Philips; Achieva Nova model, SENSE XL TORSO external coil, The Netherlands). For the examination, with no need for prior rectal cleansing nor intravenous contrast administration, patients are asked to void just before imaging, so as to have their bladder empty; then, they are placed in the supine position on the diagnostic table with their underclothing removed and a modified 3 mm wide rubber catheter is positioned intraanally to act as marker (Figure 2). The pelvic anatomy is depicted firstly with images obtained in the midsagittal plane, using the turbo spin-echo (TSE) T2-weighted pulse sequence ( TR, 4630 msec; TE,90 msec; flip angle 90°; 4 mm thick sections, 444/310 matrix and four averages; FOV, 350 mm; acq. time 3.37 min; total images 35 ). Thereafter, focusing on the intra-anal marker, the sequence is repeated with use of the same parameters in the true midcoronal and midaxial (oblique) planes taken parallel and perpendicular, respectively, to the long anal axis, for evidence of various components of the anal sphincter complex, without the distortion of the anatomy caused by the use of internal coils. [3] Finally, the short tau inversion recovery (STIR) pulse sequence is employed (TR, 2768 msec; TE, 30 msec; TI, 140 msec; flip angle, 45°; 4 mm thick sections; 512 matrix and three averages; FOV 360; acq. time 4.03 min; total images 25) using exactly the same planes. When necessary, for better depiction of the internal opening, after completion of the MR study, patients are transferred to the X-ray diagnostic room to have their rectal ampulla opacified with no more than 50 ml of diluted radiopaque contrast medium. In doing so, even minimal passage of contrast into adjacent organs is depicted at best, either at rest or during evacuation (Figure 3), an examination defined as “evacuation sinography”. A complete summary of the MR imaging protocol used is presented in Table 1.
| Parameter | Series 1 | Series 2 | Series 3 | Series 4 | Series 5 | Series 6 |
|---|---|---|---|---|---|---|
| Pulse Sequence | FSE T2-W | FSE T2-W | FSE T2-W | STIR | STIR | STIR |
| Plane | Mid Sagittal | Coronal | Axial | Mid Sagittal | Coronal | Axial |
| Oblique | Oblique | Oblique | Oblique | |||
| TR (ms) | 4435 | 3649 | 4656 | 2768 | 2768 | 2768 |
| TE (ms) | 100 | 100 | 100 | 30 | 30 | 30 |
| TI (ms) | 140 | 140 | 140 | |||
| BW | 253,0 | 172.6 | 252.4 | 1225.5 | 1225.5 | 1041.7 |
| ETL | 18 | 18 | 18 | 17 | 17 | 17 |
| NEX | 4 | 4 | 4 | 3 | 3 | 3 |
| FOV (FH-RL-AP) | 350*250*177 | 350*250*138 | 350 | 360 | 360 | 360 |
| Matrix | 560 | 560 | 560 | 512 | 512 | 512 |
| Slice/gap (mm) | 3,70– 0,13 | 3,5–0,35 | 3,5 – 0,35 | 4 | 4 | 4 |
| Flip angle (°) | 90 | 90 | 90 | 45 | 45 | 45 |
| Scan time (sec) | 2.28 | 3 | 3.37 | 2.23 | 4.03 | 3 |
| Slices (n°) | 35 | 35 | 35 | 22 | 22 | 22 |
| Fold over direction | F → H | R → L | R → L | R → L | R → L | A → P |
Table 1: Protocol for MR Imaging of anal fistula disease by Philips Achieva Nova scanner (1.5 T) and SENSE XL TORSO coil. Notes F=foot; H=head; R=right; L=left; A=anterior; P=posterior; FSE=fast spin echo; T2-W=T2-weighted; STIR=short tau inversion recovery.
Image analysis
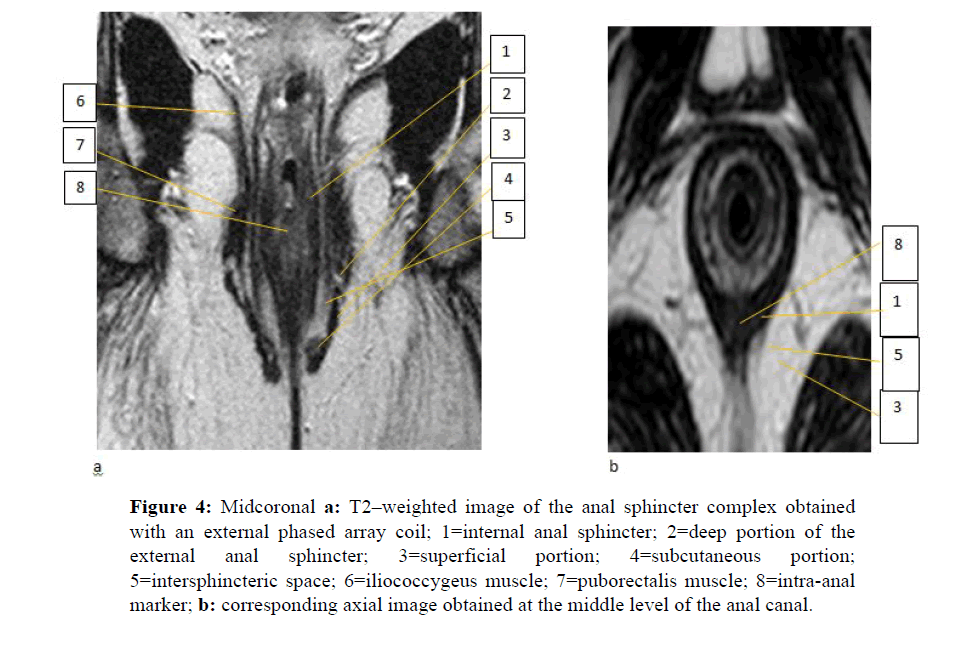
All examinations are taken to a viewing station and systematically reviewed (P.V.) for the integrity (or lack of it) of the anal sphincter complex, presence and localization of collections and sinus tracks. Thanks to the intraluminal catheter, which acts as a consistent marker while also offering a certain reference of the anal verge position, both the internal and external anal sphincters are clearly depicted on their true coronal and axial plane (Figure 4) and analyzed according to the basic criteria described by De Souza and Hussain. [3-5] On midcoronal sections the internal anal sphincter is seen as the innermost muscle layer showing an intermediate signal intensity and a longitudinal cylindrical shape, with an average thickness of 2.5 mm (range, 1.8-4 mm); just external to it, the hypointense external anal sphincter is found to exhibit an average thickness of 2.5 mm (range 1-4 mm) and a craniocaudal length of 27.0 mm. Usually, the well-known subdivision of the external sphincter into three contiguous components can be appreciated, as follows: at its cranial extent, a cleft is consistently seen between the deep portion of the sphincter and the puborectal muscle; at the caudal extremity, the subcutaneous portion is easily recognized due to its typical hooked geometrical configuration pointing medially and upward; finally, the superficial portion of the sphincter is just seen as the segment in between the two. Sometimes, a subtle difference in signal intensity between the internal and the external anal sphincters allows recognition of the inter sphincteric space, in which the longitudinal muscle layer is occasionally visible as a continuation of the outer longitudinal smooth muscle of the rectum. The whole sphincter complex is embedded in the hyperintense, fat-containing ischioanal space, while the funnel-shaped levator ani muscle separates it (below) from the supralevator space (above) where the rectal ampulla is located. On axial sections the upper, middle and lower parts of the anal canal are arranged in concentric rings of different signal intensity, depending on the relative type and amount of muscle fibers which appear from inside to outside, as follows: at the upper level, the intermediate signal intensity of the internal anal sphincter is sequentially encircled by the intersphincteric space, which contains the hypointense longitudinal muscle and by the hypointense puborectal muscle; at the middle level, all components are seen embedded into the hyperintense fat of the ischioanal fossa including the internal anal sphincter, the intersphincteric space, the external sphincter and the puborectal muscle; sections through the lowest level of the anal canal show only the two halves of the external sphincter which become progressively distant one-to- another posteriorly. On midsagittal sections, the anococcygeal ligament connects the posterior aspect of the external sphincter to the coccygeal spine and separates the deep (above) from the superficial (below) post-anal space. More laterally and anteriorly, the bulbocavernous and puborectal muscles travel toward the pubic bone. For proper identification of the fistula-in-ano disease all three scan planes are considered informative.
Figure 4: Midcoronal a: T2–weighted image of the anal sphincter complex obtained with an external phased array coil; 1=internal anal sphincter; 2=deep portion of the external anal sphincter; 3=superficial portion; 4=subcutaneous portion; 5=intersphincteric space; 6=iliococcygeus muscle; 7=puborectalis muscle; 8=intra-anal marker; b: corresponding axial image obtained at the middle level of the anal canal.
Characterization of disease
Localization: The prime aim of using MRI instead of endosonography, which has been proved to be a less accurate and less widely available technique, is to give evidence of presence and site of any collection and primary track, distance from the anal verge to fistula, position of internal and external openings and subcutaneous or supralevator extensions. Such changes can involve the intersphincteric space and remain contained within it, or extend to the ischio-recto-anal fossae, cross the levator plate and determine a collection in the rectal wall. Defining site and direction of fistulous track is usually done by referring to the “anal clock” system, that is the view of the anal region with the patient lying supine: at 12 o’clock is the anterior pubic region and at 6 o’clock the natal cleft; 3 o’clock refers to the patient left side, and 9 o’clock refers to the right side. The technique above described is of value mainly because it utilizes anatomical landmarks familiar to both the radiologist and the surgeon, making it easy to analyse images and discuss the optimal surgical strategy.
Disease activity: Overall, while active abscesses and fistula tracts have high signal intensity on both T2-weighted and STIR pulse sequences, established scars have low signal on T2 images, so that they can easily be differentiated one from another. More precisely, pathologic processes include fluid collections and primary or secondary tracks, showing high signal intensity in contrast with lower signal intensity of the sphincters, muscles and fibrotic changes. Occasionally, the hyperintense signal intensity from the fat of the ischio-anal fossa makes it somewhat difficult to detect the precise contours of the abnormality in singular cases. The corresponding STIR images will help the examiner to depict both the true boundaries and the activity of the disease. Nevertheless, some pitfalls should be taken in mind, [6] such as (a) retained pus material remaining unenhanced after contrast administration within the walls of abscess cavities, with resulting peripheral “ring” effect; and (b) fat containing “grafts” used by surgeons to fill cavities. Moreover, STIR images are sometimes unable to distinguish small abscesses from perianal inflammation and may show spurious high signal in old fibrotic tracks.
Results
The MRI examination was well tolerated by all the thirty patients who in no case denied their consent to insertion of the catheter used as intra-anal marker, thanks to its small size and safety, even in the presence of significant local pain or recent surgery. This proved critical in depicting at best the MR anatomy of the region of interest (ROI) in its entirety from inside the anal lumen down to the skin of the gluteal margin (bottom), up to the recto-sigmoid junction (top), and laterally to the obturator internus muscle of each side. The average time interval from the onset of symptoms to the request of MRI study was 13 ± 2 months (range 3-39 months), while in no more than 5 out of 30 cases (16.6%) patients exhibited an ultrasonographic series, probably reflecting both limited availability of the endoanal technique throughout the country and lower patient acceptance for fear of pain. Overall, the diagnostic yield of the MRI examination added new significant information (Table 2) which significantly altered the relative proportion between simple vs complex anal fistula diagnoses and the subsequent management, leading to a dramatic increase (+440%) in the rate of complex surgery, i.e. need for reoperation and/or a more aggressive and extensive surgical procedure, in 27 out of 30 subjects (90%). Most interestingly, unsuspected “complex disease “parameters at MRI were found in those cases regardless of the time interval from the onset of symptoms and performance of the test. As expected, when compared to physical examination with or without three dimensional 3D endoanal-ultrasonography (EAUS), the information gained from the MRI test most frequently regarded the diagnosis of persistent disease activity, despite apparent healing (83.3%) and unknown inflammatory reaction, affecting the fat tissues of the ischio-anal fossae (73.3%), both of which are unique prerequisites of MRI and in no case were carried out at sonography. Other findings included distant extension to adjacent structures and additional tracts (23.3% each). Although less frequently seen (13%), supralevator abscesses including those located within the rectal wall were diagnosed at MRI only. On the other hand, a similar accuracy at ultrasonography and MRI was registered in the identification rate of horseshoe abscesses, number and location of internal and external openings and subcutaneous collections (23.3%, 33.3%, and 20%, respectively).
| Diagnostic Tool | N° | Fistula Classification Simple Complex | Change (%) in surgical planning Simple Complex |
|---|---|---|---|
| Before MRI Clinical evaluation 3-D EAUS |
30 5 |
25 5 1 4 |
-20 |
| After MRI (external coil) |
30 | 3 27 | -88 +440 |
Table 2: Effect of MR Imaging vs clinical evaluation with or without EAUS on operative planning in thirty consecutive patients with anal fistula disease. Note and Complex parameters at MRI most frequently included: disease activity despite apparent healing (83.3%); inflammatory reaction of fat tissues (73.3%); additional tracks and distant extensions (23.3% each).
Spectrum of Abnormalities
The following are three examples taken from the series of the present population; they highlight the expanding clinical utility of MRI [7] in the diagnosis and treatment of fistula-in- ano disease which in turn consist of detecting undiagnosed extensions and providing the optimal surgical road map. All this sounds as an overt encouragement to consider the role of MRI before any other imaging modality in the diagnostic work-up of ano-perianal sepsis.
Case 1
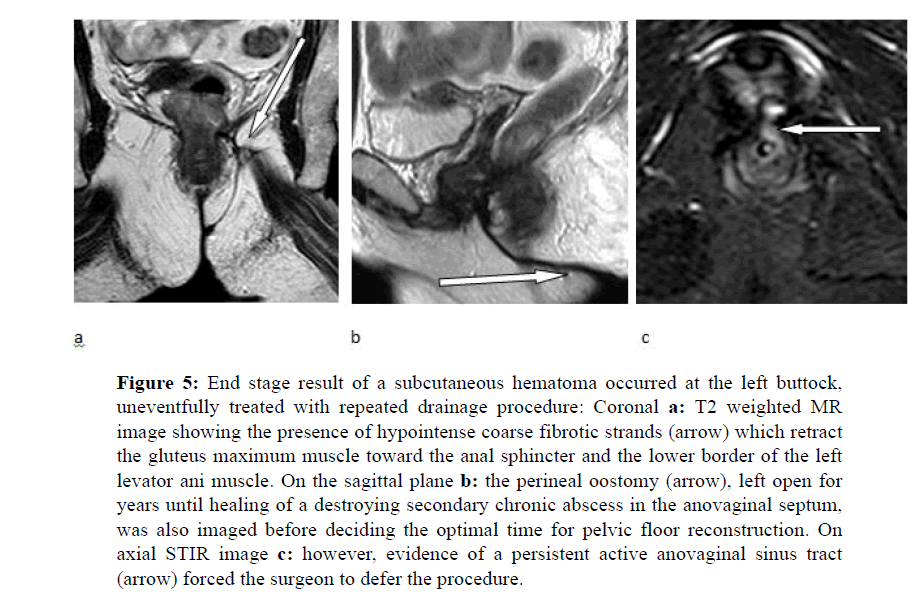
A fifty-one year-old woman with old history of accidental trauma on her left buttock which occurred eleven years before during a jeep trip in the Egyptian desert; one month later, back to Italy, she began to complain fluctuant, subcutaneous tenderness which was found at sonography to be due to fluid collection. After drainage, at pathology, the material was consistent with hematoma and crashed fibro fatty tissues. Few days later, however, due to recurrence of symptoms, she underwent new drainage sessions, which resulted in lack of healing and development of extensive abscess within the rectovaginal septum, secondary anovaginal fistula and sphincter damage, despite combined medical and surgical therapy, being continuously checked through multiple fistulographic imaging series and even CT examinations. Currently the patient, bearer of wide postoperative perineal oostomy (Figure 5) between the vaginal opening and the anal verge, was sent by the referring surgeon to undergo MR examination for a reported sensation of passage of air into the vagina and increased buttock pressure exacerbated from sitting.
Figure 5: End stage result of a subcutaneous hematoma occurred at the left buttock, uneventfully treated with repeated drainage procedure: Coronal a: T2 weighted MR image showing the presence of hypointense coarse fibrotic strands (arrow) which retract the gluteus maximum muscle toward the anal sphincter and the lower border of the left levator ani muscle. On the sagittal plane b: the perineal oostomy (arrow), left open for years until healing of a destroying secondary chronic abscess in the anovaginal septum, was also imaged before deciding the optimal time for pelvic floor reconstruction. On axial STIR image c: however, evidence of a persistent active anovaginal sinus tract (arrow) forced the surgeon to defer the procedure.
Case 2
A sixty year-old man with no history of prior anorectal disease nor evacuation dysfunctions, experienced an episode of acute bilateral retroanal pain. At ano-proctoscopy, a definite intraluminal bulging of the right and left anal walls was detected which was subsequently proved at endoanal ultrasonography to be consistent with the presence of a horseshoe intersphincteric abscess. At surgery, abundant pus material was drained and two loose setons were left in place on each side of the retroanal space. Five months later, however, due to persistent discharge, the patient was submitted to a new surgical drainage procedure. Currently, one year after the onset of symptoms and still occasional discharge, the surgeon eventually decided to refer him to the radiology department for MR imaging examination (Figure 6).
Case 3
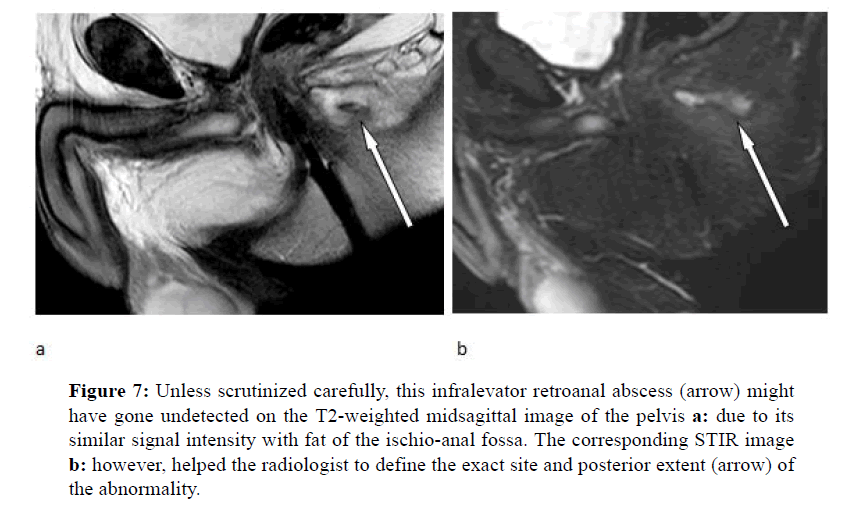
A sixty-nine year-old man with prior right hemicolectomy due to colonic cancer set in seven years before complained sudden perianal pain suggesting a fistula-in-ano disease. With no other evaluation than a digital rectal examination (DRE), suspecting an abscess, the consultant surgeon proposed to treat it surgically. This was actually found and successfully drained via the percutaneous route resulting in apparent healing. One month later, the patient was referred to MR imaging study just for routine post-operative followup (Figure 7).
Figure 7: Unless scrutinized carefully, this infralevator retroanal abscess (arrow) might have gone undetected on the T2-weighted midsagittal image of the pelvis a: due to its similar signal intensity with fat of the ischio-anal fossa. The corresponding STIR image b: however, helped the radiologist to define the exact site and posterior extent (arrow) of the abnormality.
Discussion
Today, many surgeons in Italy still have a wrong conception of the use of imaging techniques in the preoperative evaluation of anal fistula disease. A recent Consensus Statement in Italy [2] affirmed that most abscesses and fistulas do not require any imaging as the diagnosis is usually made on the basis of the patient’s history and physical examination. According to the authors, while endoanal ultrasound is the first-line imaging in complex fistula (Grade of recommendation 1B), X-ray fistulography is not suggested for the diagnosis since it has a low accuracy and may be poorly tolerated by the patient. With regard to magnetic resonance imaging (MRI), this should be considered in selected patients only and in complex cases in order to detect occult abscess and secondary tract formation or to assess the integrity and function of the sphincter muscles. Recently, however, the behavior of many young surgeons hs been rapidly changing and no longer it is observed such a delay (see case 1) from the onset of symptoms to the request of the MR imaging study. Rather, patients are referred to it as soon as possible in case of suspect of persistent disease activity (see case 2) and even as routine follow-up to confirm the apparent healing (case 3). Certainly, it can be affirmed that the advent of fast MR imaging in the early 90s has radically changed the approach of modern medicine to the assessment and treatment of ano-perianal sepsis with no need for contrast administration, unlike CT, or sophisticated imaging technique. [8,9] More specifically, technical advances registered in the field of image acquisition ÃÆâÃâââ¬Ãâââ¬Â¢ up to 16 times faster than with the standard Spin echo (SE) pulse sequence ÃÆâÃâââ¬Ãâââ¬Â¢ make MRI the ideal modality to depict the pelvic floor anatomy in its entirety. Hopefully, the common embarrassment of most surgeons faced with the difficult task of reading and interpreting such a “huge amount” of images yielded during scanning, is expected to disappear if the radiologists limit their attitude, when producing their report, to offer an unfruitful description of changes and to highlight the “technical” aspects rather than the clinical relevance of the examination. With reference to this issue, it may be worth including in the report a schematic drawing with labels indicating the exact location and extent of the changes found in singular cases. The present paper is an attempt to improve the dialogue and the trend to exchange knowledge between surgeons and radiologists, in order to obtain better treatment of perianal sepsis and reduced recurrence rate.
Conclusion
Although the great majority of patients with perianal sepsis will continue to be adequately treated by incision and drainage, MR imaging is assuming a new important and expanding role today, should this technique be taken into consideration at the very beginning of disease’s clinical manifestation. The present paper offers an evidence of the clinical efficacy of the technique and suggests the potential advantage of inverting the current generally accepted diagnostic algorithm which recommends performing EAUS as the first imaging modality. Future studies will tell us if trends observed here will continue to be of any value.
References
- Beets-Tan RG., Beets GL., van der Hoop. Preoperative MR imaging of anal fistulas: does it really help the surgeon? Radiology 2001; 218: 75-84.
- Amato A., Bottini C., De Nardi P. Evaluation and management of perianal abscess and anal fistula: a consensus statement developed by the Italian Society of Colorectal Surgery (SICCR). Tech Coloproctol 2015; 19: 595–606.
- deSouza NM., Puni R., Gilderdale DJ. Magnetic resonance imaging of the anal sphincter using an internal coil. Magnet Res Quart 1995; 11: 45-56.
- Hussain SM., Stoker J., Lameris JS. Anal sphincter complex: endoanal MR Imaging of normal anatomy. Radiology 1995; 197: 671-677.
- deSouza NM., Gilderdale DJ., Coutts GA. MRI of fistula-in-ano: a comparison of endoanal coil with external phased array coil techniques. J Comp Assist Tomogr 1998; 22: 357-363.
- Morris J., Spencer JA., Ambrose NS. MR Imaging classification of perianal fistulas and its implications for patient management. Radiographics 2000; 20: 623-635.
- Piloni V., Pescatori M. Evaluation of recurrent and/or persistent complex ano-perianal abscess after surgery: the unique value of magnetic resonance (MR) imaging. IJCMI 2015; 2: 2376.
- Changhu L., Yongchao L., Bin Z. Imaging of anal fistulas: comparison of computed tomographic fistulography and magnetic resonance imaging. Korean J Radiol 2014; 15: 712-723.
- Schafer O., Lohrmann C., Langer M. Digital subtraction MR fistulography: new diagnostic tool for the detection of fistula in ano. AJR 2003; 181: 1611-1613.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences