Optimisation of Europium Sensitized Fluorescence Assay for Detection of Tetracycline Antibiotics
Ervina BeÄÂÂić, Jasmin Mušanović, Belma Imamović, Fahir BeÄÂÂić, Miroslav Šober
1Department of Pharmaceutical Analysis, University of Sarajevo, Bosnia and Herzegovina
2Department of Biology, University of Sarajevo, Bosnia and Herzegovina
3Department of Clinical Pharmacy, University of Sarajevo, Bosnia and Herzegovina
- *Corresponding Author:
- Ervina Bečić
Department of Pharmaceutical Analysis
University of Sarajevo, Bosnia and Herzegovina
Tel: 38733586179
E-mail: ervinafarmacy@gmail.com
Abstract
Objective: Europium sensitized fluorescence method for tetracycline antibiotics determination in various samples was optimized. Method: The factors that influence intensity of the europium–tetracycline fluorescence have been studied in detail. Fluorescence intensity measurement was carried out using Shimadzu RF-5301-PC spectrofluorometer (Kyoto, Japan) with Panorama fluorescence 1.1 software. Results: The parameters that can affect the fluorescence of europium-tetracycline complex are optimized. Best results were obtained using tris buffer and 1 mm of citric acid as co ligands. The method is linear in the range of 5-2500 ng/ml for tetracycline and oxytetracycline and 5-1000 ng/ml for chlortetracycline and can be used for their determination in various samples. Conclusion: Europium–tetracycline sensitized fluorescence is a very sensitive that can be used for determination very low tetracycline concentrations. It is necessary to optimize all parameters that can affect the fluorescence, in order to achieve low detection limits.
Keywords
Tetracycline, Resistence, Europium sensitized fluorescence, Method optimisation
Introduction
Today, human and veterinary medicine uses more than 250 different antibiotics and antimycotic agents in the treatment of a large number of infectious diseases [1]. It is known that about 50% of the total amount produced antibiotics is used in veterinary medicine and for the growth stimulation [2]. Many antibiotics are excreted unchanged or as metabolites in the faeces and urine during the grazing animals and are due directly to agricultural land. Depending on the chemical properties of individual groups of antibiotics excretion of unchanged active substance is 10-90% [3,4]. The widespread use of antibiotics in medicine and animal husbandry is the most important factor for the emergence, selection and dissemination of antibiotic resistant bacteria [5-7].
Tetracycline (TC), oxtetracycline (OTC) and chlortetracycline (CTC) which is a kind of tetracycline antibiotics group were long time, the most used throughout the world [8-10]. They have been widely used for the past sixty years as therapeutic agent in human and veterinary medicine and also as growth promotor in animal husbandry. Their use for humans was nowadays limited due the emergence of bacterial resistances. In human medicine, tetracyclines have been prescribed for prophylaxis and treatment of respiratory infection. They have been the drugs of choice for treatment of pneumonia due to Mycoplasma pneumoniae, Chlamydia pneumoniae, and Chlamydia psittaci [11]. Antibacterial activity of typical tetracyclines is associated with the reversible inhibition of the protein synthesis, but bacterial resistance to tetracycline was identified shortly after the introduction of therapy [12]. The first tetracycline resistant bacterium was Shigella dysenteriae isolated in 1953 [13]. Three different specific mechanisms of tetracycline resistance have been descriebed: tetracycline efflux, ribosomal protection as the most common resistance mechanisms and tetracycline modification-enzymatic inactivation [14].
Presence of antibiotic resistant genes, such as TET or OTET genes, have been reported in wastewater, surface water and sediments [15-17]. Tetracyclines are strong complexing agents that easy bind with metal ions as calcium or magnesium from water or soil, which can reduce their mobility in the environment, and therefore the detection [18].
There are numerous literature data that describe methods for the identification and quantification of tetracyclines antibiotics in various samples [19-22]. They forms fluorescent complex with the europium ion via intramolecular energy transfer from the ligand to europium, which yields a characteristic emission at 619 nm [23]. Europium–tetracycline fluorescence intensity depends on the concentration of metals and ligands, use of the coligands or surfactants and pH and is therefore necessary to optimize the conditions to maximize the sensitivity of the method [24].
Materials and Methods
All reagents used were analythical grade. Tetracycline, Oxytetracycline and Chlortetracycline (Sigma) was min. 98% pure. Ultra-pure water was used throughout. Stock solutions were prepared by dissolving accurate quantities of the powdered standards in 1 ml ultra-pure water and then diluted with acetonitrile. Mass concentration of standard solutions was 500 μg/ml. Stock solutions stored were protected from light at 40C. Working standard solutions were made by diluting the stock standard solutions with acetonitrile in concentration range 5–2500 ng/ml. Stock solution of europium (1,6 mm) was prepared from EuCl3 × 6H2O (Aldrich). Citric acid, oxalic acid and tartaric acid stock solutions (1 mm) were prepared by dissolving appropriate amount of powdered substances in ultra-pure water. Tris and borate buffer solutions pH 6-9 were prepared in ultra-pure water according the Ph. Eur. procedure.
Fluorescence intensity measurement was carried out using Shimadzu RF-5301-PC spectrofluorometer (Kyoto, Japan) with Panorama fluorescence 1.1 software.
Results
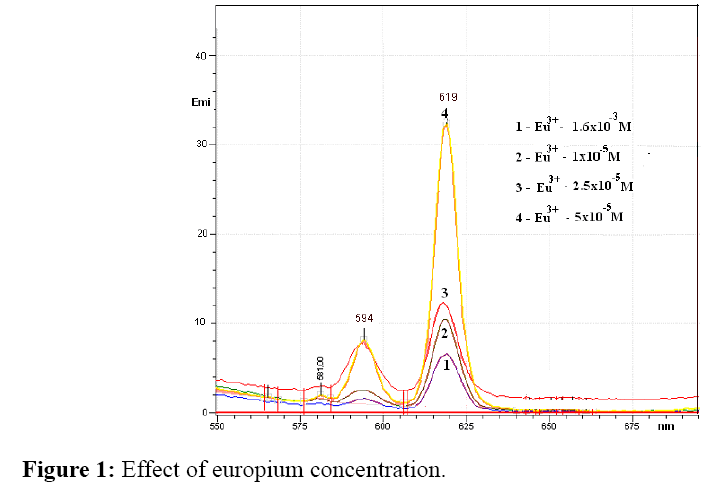
Effect of europium (III) concentration
The influence of europium (III) concentration was determined by measuring the fluorescence intensity at a concentration of europium (III) 1 × 10-6 M, 2.5 × 10-6 M, 5 × 10-5 M and 1.6 × 10-3 M, wherein the concentration of the antibiotic and buffer pH was kept constant.
Figure 1 show the fluorescence spectra of europium complexes with different europium (III) concentrations. As can be seen, the lowest fluorescence intensity was with highest europium concentration. By reducing the concentration, fluorescence intensity increase in follow order 5 × 10-5 M>1 × 10-5>2.5 × 10-6 M. In accordance with obtained results europium concentration 5 × 10-5 M was chosen for further work.
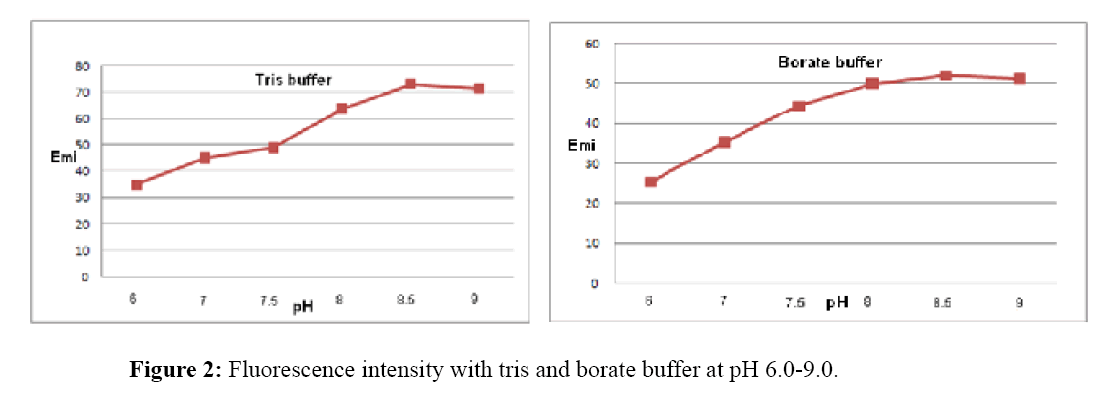
Effect of pH and buffer solution
Figure 2 show the results of buffer selection. Tris buffer and borate buffer with different pH values (6.0, 7.0, 7.5, 8.0. 8.5, 9.0), were tested.
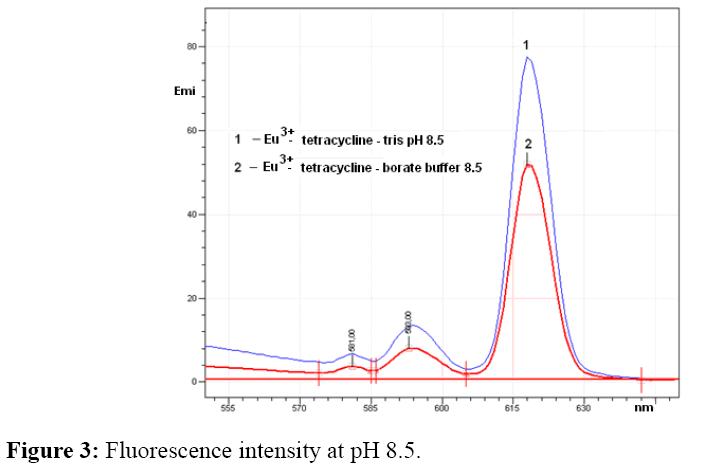
Emission intensity of europium-tetracycline complex with both tested buffer was very similar, increases to pH 8.5, than for the both buffer begins to drop. Fluorescence intensity with tris buffer, increases by about 30% at the same pH values (Figure 3). For further measurement tris buffer pH 8.5 was used.
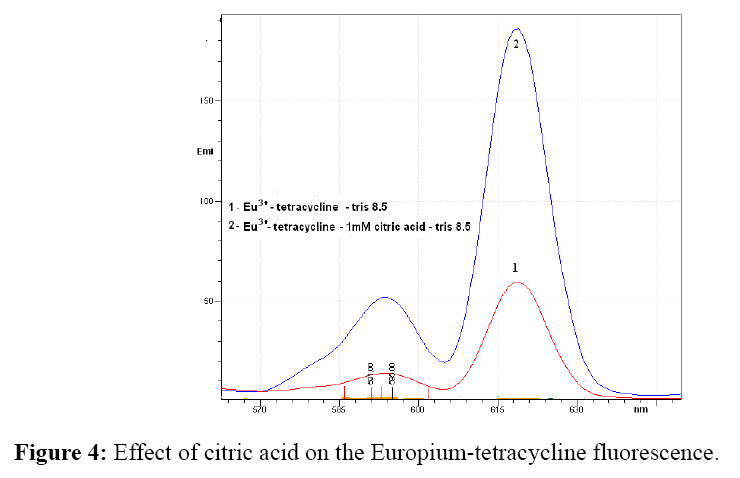
Effect of coligands
The influence of bidentate ligands oxalic and tartaric acid and polydentate ligand citric acid on the fluorescence intensity of europium-tetracycline complex, were also investigated. An increase in fluorescence intensity occurs only in the presence of citric acid.
As can be seen from Figure 4 fluorescence intensity increased about 3.5 times if added citric acid to mixture of europium (III) and tetracycline antibiotics.
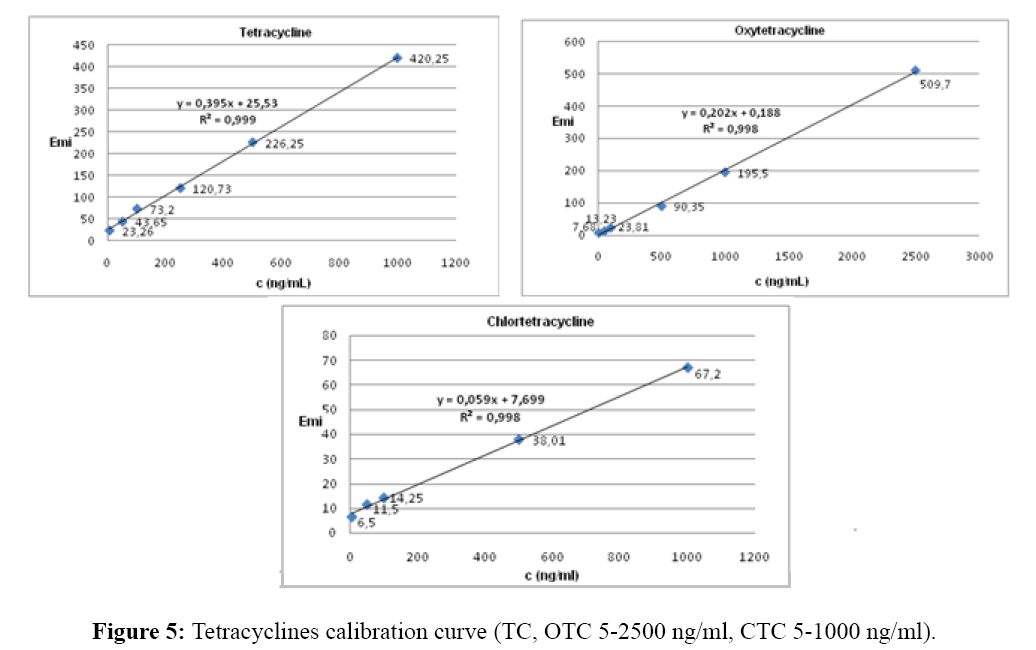
Effect of antibiotic concentrations
The optimum antibiotic concentration was investigated in the range of 5-2500 ng/mL of each antibiotic at a constant concentration of europium (III), 1 mM citric acid and tris buffer pH 8.5. The linear relationship of the fluorescence intensity and the antibiotic concentration usefull for analytical purposes was 5-2500 ng/ml for TC and OTC and 5-1000 ng/ml for CTC, respectively. Corellation coefficients were 0.999 for TC and 0.998 for OTC and CTC (Figure 5).
Discussion
The emission spectrum of europium-tetracycline complex show a maximum emission characteristic for 5d0 → 1F7 at 590 nm, and 5d0 → 2F7 at 619 nm. The 5d0 → 2F7 transition is strongest (red-orange emission) and the emission maximum at 619 nm is used fluorescence measurement [25]. The fluorescence of the europium complex is very sensitive.
Low reduction potential of europium cause non-radiative energy transfer from the ligand to the metal with a significant decrease in fluorescence intensity [25]. It is therefore necessary to optimize the parameters for fluorescence useful in analytical measurement.
Earlier investigations showed that fluorescence of europium complex dependence of the europium concentration, concentration of ligands, coligands and pH [26].
Tetracyclines show fluorescence in a neutral media. In basic media fluorescence considerably increased due to the deprotonation electron donor group [24]. Considering this fact, the influence of tris and borate buffer at pH 7.0, 7.5, 8.0. 8.5 and 9.0 were studied.
It was found that tris buffer increases fluorescence, probably because it penetrates the coordination sphere of europium, acting synergistically and in the same time reducing the impact of water on the complex [27]. In this case, its function is to achieve the optimal pH for the formation of the complex and the fluorescence.
As can be seen from Figure 3 the increase of pH of tris and borate buffer to 8.5 increase the fluorescence intensity of the europium–tetracycline complex. It can be explained by deprotonating of tetracycline molecule or groups involved in the formation of complex. Tetracycline forms complex with multivalent cations over tricarbonyl or β-diketone ring, which become nucleophilic after deprotonation. At pH 9.0 the fluorescence intensity begins to drop, so that the pH 8.5 can be considered as optimal for use in analytical purposes. To increase the fluorescence intensity of europium tetracycline complex, is possibile to use co-ligand such neutral, anionic or cationic surfactants [25]. In this study were investigated influence of citric, oxalic and tartaric acid as co-ligands. The obtained results showed that an increase in fluorescence intensity occurs only in the presence of citrate.
Oxalate has no effect on the fluorescence intensity of the europium-tetracycline complex at 619 nm and the fluorescence originating from 5d0 → 7F2 transitions, however, the intensity of fluorescence derived from 5d0 → 7F1, is about 25% higher. The tartrate increases the fluorescence intensity at 619 for about 20% and at 594 nm fluorescence is 20% lower. In contrast, with citric acid the fluorescence intensity was 3.5 times higher for both emission maximum, as europium – tetracycline fluorescence. With three oxygen atoms of the carboxyl groups and the oxygen of the hydroxyl group citrate is coupled with europium and removes the water molecules that occupy the coordination sphere of the europium ion, thereby forming a new complex of europium- tetracycline-citrate. In the europium-tetracycline-citrate complex, both, the tetracycline and citrate are protonated and sensitive to pH. Tetracycline has three pKa values, about 3.3, 7.7 and 9.5 and citric acid 3.1, 4.7 and 6.4. However, the formed complex is stable in a broad pH range from 7.4 to 9.225. As the pKa of oxalic (1.23 and 4.19) and the tartaric acid (3.03 and 4.37) are lower than pKa values of citric acid it is possible that protonation at pH 8.5 is only partially, which results in limited binding to europium ion and weak increase in emission intensity. This data is very usefull for further use of europium–tetracycline– complex especially for the possibile development and optimization of the HPLC method. It is known that mobile phase for tetracycline chromatography, very often used, some of the previously investigated acid.
For the practical analytical use the linear relationship of the fluorescence intensity and the tetracyclines (TC; OTC; CTC) concentration were established in the range of 5-2500 ng/ml for TC and OTC, and 5-1000 ng/ml for CTC.
Conclusion
Europium–tetracycline sensitized fluorescence is a very sensitive and therefore very applicable for the tetracycline antibiotics determination in very low concentrations. It is necessary to optimize all parameters that can affect the fluorescence, in order to achieve low detection limits.
Linearity in a wide range of concentrations and good correlation coefficient can be the basis for the development and validation of analytical methods for the determination of tetracycline antibiotics in different samples.
References
- Kummerer K. Pharmaceuticals in the Environment. Springer-Verlag 2004; 2nd ed.
- Boxall ABA., Fogg LA., Kay P., Blackwell PA., Pemberton EJ., et al. Veterinary medicines in the environment. Rev Environ Contam T 2003; 180: 1-91.
- Hektoen H., Berge JA., Hormazabal V., Yndestad M. Resistance of antimicrobial agents in marine sediments. Aquaculture 1995; 133: 175-184.
- Serrano PH. Responsible use of antibiotics in aquaculture, FAO fisheries technical paper. Rome, Italy: Food and Agriculture Organization of the United Nations2003.
- Adam D. Global antibiotic resistance in Streptococcus pneumoniae. J AntimicrobChemoter 2002; 50: 1-5.
- McDonald LC., Kuehnert MJ., Tenover FC., Jarvis WR. Vancomycin-resistant enterococci outside the health-care setting: prevalence, sources and public health implications. Emerg Infect Dis 1997; 3: 311-317.
- Sarmah AK., Meyer MT., Boxall AB. A global perspective on the use, sales, exposure patways, occurrence, fate and effects of veterinary antibiotics (Vas) in the environment. Chemosphere 2006; 65: 725-759.
- LópezÃÆâÃâââ¬ÃâÃÂPeñalver JJ., SánchezÃÆâÃâââ¬ÃâÃÂPolo M., GómezÃÆâÃâââ¬ÃâÃÂPacheco CV. Photodegradation of tetracyclines in aquous solution by using UV and H2O2 oxidation process. J Chem Tech Biotech 2010; 85: 1325-1333.
- Jeong J., Song W., Cooper WJ., Jung J., Greaves J. Degradation of tetracycline antibiotics: Mechanisms and Kinetic studies for advanced oxidation/reduction processes. Chemosphere 2010; 78: 533-540.
- Halling-Sørensen B., Sengeløv G., Tjørnelund J. Toxicity of tetracyclines and tetracycline degradation products to environmentally relevant bacteria, including selected tetracycline-resistant bacteria. Arch Environ ContamToxico 2002; 42:263-271.
- Chopra I., Roberts MC. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. MicrobiolMolBiol Rev 2001; 65: 232-260.
- Roberts MC. Tetracycline Therapy: Update. Clin Infect Diseases 2003; 36:262-267.
- Michalova E., Novotna P., Schlegelova J. Tetracyclines in veterinary medicine and bacterial resistance to them. Vet Med – Czech 2004; 3: 79-100.
- Falkow S. Infectious multiple drug resistance. Pion1975.
- Speer BS., Bezdyk L., Salyers A. Evidence that a Novel Tetracycline Resistance Gene Found on Two Bacteroides Transposons Encodes an NADP-Requiring Oxidoreductase. J Bacter 1991; 173: 176-183.
- Schwartz T., Kohnen W., Jansen B., Obst U. Detection of antibiotic-resistant bacteria and their resistance genes in wastewater, surface water, and drinking water biofilms. FEMS MicrobiolEcol2003; 43: 325-335.
- Pei R., Kim SCH., Carlson KH., Pruden A. Effect of River Landscape on the sediment concentrations of antibiotics and corresponding antibiotic resistance genes (ARG). Wat Res2006; 40: 2427-2435.
- Ram S., Vajpayee P., Shanker R. Prevalence of multi antimicrobial agent resistant, shiga toxin and enterotoxins producing Escherichia coli in surface water of the river Ganga. Environ Sci Tech2007; 41: 7383-7388.
- Prasad AR., Rao VS. Spectrophotometric methods for the microdetermination of oxytetracycline and hostacycline. Sci World J 2010; 5: 1-4.
- Thiex NJ., Larson R. Determination of oxytetracycline /oxytetracyclinehydrochlotide in animal feed, fish feed, and veterinary medicinal products by liquid chromatography with fluorescence detection: collaborative study. J AOAC Int 2009; 92: 2-14.
- Abbasia MM., Nematia M., Babaeia H., Ansarina M,, NourdadgarAoS. Solid-Phase Extraction and Simultaneous Determination of Tetracycline Residues in Edible Cattle Tissues Using an HPLC-FL Method. Iran J Pharm Res 2012; 11: 781-787.
- Calixto CMF., Cervini P., Cavalheiro ETG. Determination of Tetracycline in Environmental Water Samples at a Graphite-Polyurethane Composite Electrode. J BrazChemSoc 2012; 23: 938-943.
- Grasso AN., Teixeira LS., Vieira ND Jr., Courrol LC. Optical properties of metacycline, oxytetracycline and chlortetracycline europium complexes in the presence of hydrogen peroxide. J Fluoresc 2009; 19: 715-721.
- Wang X., Zhao H., Jin L. Determination of Eu(III) based on fluorescence and cofluorescence enhancement of Eu(III)-Tb(III)-tetracycline-citrate system. Ind J Chem 2007; 46A: 1801-1804.
- Courrol LC., Samad RE. Applications of Europium Tetracycline Complex: A Review. Curr Pharm Anal2008; 4:238-248.
- Lis S., Elbanowsli M., Makowska B., Hnatejko Z. Energy transfer in solution of lanthanide complexes. J PhotochemPhotobiol A Chemistry2002; 150: 233-247.
- Goèmez-Hens A., Aguilar-Caballos MP. Terbium-sensitized luminescence: a selective and versatile analytical approach. Trends AnalytChem 2002; 21: 131-141.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences