Investigation of Genotoxicity of Homosalate Byproducts Occured in the Presence of Free Chlorine by Using Allium Test
Belma Imamović, Jasmin Mušanović, Ervina Bečić, Miroslav Šober
Belma Imamović1*, Jasmin Mušanović2, Ervina Bečić1, Miroslav Šober1
1Department of Pharmaceutical Analysis, University of Sarajevo, Bosnia and Herzegovina
2Department of Biology, University of Sarajevo, Bosnia and Herzegovina
- *Corresponding Author:
- Belma Imamović
Department of Pharmaceutical Analysis
University of Sarajevo, Bosnia and Herzegovina
Tel: 38761374942
E-mail: belma_i@yahoo.com
Abstract
Objective: The aim of this study was to investigate the genotoxic effects of chlorinated homosalate by-products occurred in the presence of free chlorine. Method: The genotoxic effects of chlorinated homosalate by-products were investigated using Allium test. Cytogenetic analysis included monitoring influence of various concentrations of the test filter (10, 30 and 50 ng/ml) at various concentrations of free chlorine (0.2, 0.4 and 0.6 mg/ml), on mitotic activity of meristem cells., as well as the types of genotoxic effects expressed during the cell cycle. The observed effects were quantitatively presented at 2000 analyzed cells. Results: Each tested homosalate concentration causes a reduction of the mitotic index (MI) comparing to the water as a control, after 24 and 48 hours exposure as well. Statistical analysis demonstrated highly significant difference (p<0.0001) between the MI of water and MI of investigated solution. Besides the water as the basic control, the comparison was also performed compared to the three controls represented the concentration of free chlorine (0.2, 0.4 and 0.6 mg/ml). Most of the investigated concentration had highly significant difference of MI if compared to MI of their control containing different concentration of free chlorine. The number of irregular phases caused by investigated homosalate solutions was determined by cytogenetic analysis. It was found that the investigated solutions in the applied concentration and at the length of treatment (24 and 48 hours), disturbed chromosomes kinetics at certain stages of cell division. Aberrations in cells of the onion root appeared in the form of chromosome kinetic disturbance, the appearance of cells with "chromosomal bridge", cells with agglutinated chromosomes and micronuclei. Conclusion: The results of this study have shown that chlorinated homosalate byproducts that occur in the presence of free chlorine, show genotoxic effects on the onion root and interfere with the normal division of meristem cells.
Keywords
Homosalate, By-products, Free chlorine, Allium test, Genotoxic effects
Introduction
A number of cosmetic products (such as lipstick, face cream, hair spray) and products for personal hygiene (such as shampoo, detergent) containing UV filters [1] have appeared on the global market in the recent time. This phenomenon is due to the increasing public concern about the harmful effects of UV radiation to human health. On the other hand, there is increasing public concern about potential harmful effects of the ingredients contained in these preparations, in case they get into the environment. The focus is on organic UV filters, which are ingredient of a number of cosmetic products. The role of UV filters is to absorb, reflect and/or scatter the UV radiation (320-400 nm UVA and 290-320 nm UVB) and thus protect against its harmful effect to human health. UV filters can be inorganic substances (also known as physical UV filters) which reflect and scatter UV radiation, or organic substances (known as chemical UV filters) which absorb UV radiation [2]. In general, these are compounds whose structures contain one or more aromatic rings, conjugated with an electron releasing and an electron receiving groups, which are in the ortho or para positions. This enables effective delocalization of electrons, which brings specific absorption maxima at certain wavelengths [3]. The highest concentrations of organic UV filters are found in sunscreen products. After dermal application, a certain amount of organic UV filter passes through the skin and is distributed throughout the body. Some of it is excreted in the original form, while some is metabolized in the body [4]. UV filters can reach the environment in different ways. These pathways can be direct or indirect [5]. Direct originate from rinsing when in oceans, lakes, rivers and swimming pools, as well as from industrial wastewater, while indirect originate from household wastewater (showers, laundry etc.) and during plants irrigation. UV filters are substances that should be stable when exposed to sunlight. However, there are studies which have investigated the stability of UV filters during their exposure to light (natural or artificial). Exposure to light resulted in photo-degradation reaction. As a result of photo-degradation, unwanted photoproducts occurred, which tended toward being accumulated on skin surface. Also, once reaching aqueous medium UV filters are degraded in the presence of sunlight. Some UV filters have the ability of photodegradation some reactive oxygen compounds and other free radicals when exposed to UV radiation in aqueous solution [6-9]. UV filters are also subjected to degradation in the presence of water disinfectants (NaOCl in swimming pools) where occurring byproducts can potentially have harmful effect on living organisms. The nature of the formation of chlorine derivatives depends on the type of organic substances and the conditions in which the reaction takes place: pH, concentration, the share of chlorine, the presence of amino compounds, etc. The concentration of residual chlorine in swimming pool water has to be kept within certain limits (0.2-0.5 mg/l). In some countries, the maximum concentration of residual chlorine (0.4 mg/l) and the pH of the pool water (7.4-7.6) are regulated by directives [6,10-14] Chlorinated by-products in the swimming pool water occurred during the reaction of residual chlorine with organic substances in water (soil, sweat, urine, organic UV filters from sunscreen). Identification of chlorinated byproducts of organic UV filters is very important due to their potentially harmful effects on the environment and human health. In most cases these by-products show stronger toxic effects than their parental UV filters [7,10-14]. As it was mentioned above, the organic UV filters and their by-products occurring under the influence of UV radiation and free chlorine can be considered as a new class of pollutants. A few papers treating this field have been published. Several studies treated genotoxicity of water disinfectants (NaOCl, ClO2). Authors Maonarca et al. at their study [15] investigated influence of different concentrations of disinfectants on the growth of Allium cepa roots. They found that these compounds have genotoxic effect on the growth of Allium cepa. In the paper by Nakajima et al [16]. two organic UV filters, octyl-4-methoxycinnamate and octyl-4- dimethylaminobenzoate have been investigated. It was observed whether these organic UV filters and their by-products occurring during the reaction with free chlorine showed genotoxic effects. It has been found that the chlorinated by-products of octyl-4-methoxy cinnamate show low genotoxicity to Salmonella typhimurium, compared to octyl 4- methoxy cinnamate itself which shows none. In recent study, [17] oxybenzone, dioxybenzone and sulisobenzone were tested on genotoxicity. Chlorinated by-products of dioxybenzone and oxybenzone showed significant genotoxicity compared to dioxybenzone and oxybenzone. On the opposite side, chlorinated by-products of sulisobenzone showed less genotoxicity then sulisobenzone itself. Genotoxic effects of commercially available sunscreen products have also been examined. It was found that sunscreen product absorbance and its UV protection are dramatically reduced if it is exposed to free chlorine and its genotoxicity [17] is increased. In order to determine the genotoxicity of organic UV filters and their by-products which occurred during the reaction with chlorine, this study conducted research of genotoxicity of homosalate and its by-products in the presence of free chlorine.
Materials and methods
Reagents, standard and materials
Homosalate standard (HS) was purchaed from Merck (Germany). A commercial sodium hypochlorite solution with chlorine content 2.5% was used in chlorination study. This solution was stored at 4oC and its exact concentration was determined by iodometric titration using standard procedures. Deionised water was used in all experiments. Equalsized bulbs (25–30 mm in diameter) of a commercial variety of Allium cepa (L. familia: Liliaceae, 2n=16) were used as the test plant.
The study of genotoxicity using Allium test
In this part of the study somewhat modified original Allium test method was used. The study was performed at the meristem cells of tip of the root of onion-Allium cepa. Wellfed bulbs with diameter 1.5-2 cm, germinated in ordinary drinking water for 2-3 days, until root reached a length of 3 cm. Bulbs (five for each concentration of tested filters and for control as well) were then transferred to test tubes filled with 10 ml of tested homosalate solution. Solutions of the tested filter were at concentration of: 10, 30 and 50 ng/ml, and each of these concentrations were prepared in three concentrations of free chlorine: 0.2, 0.4 and 0.6 mg/ml. The solution preparation procedure in order to analyze and indentify homosalate chlorine by-products is shown in previous paper [18]. Treatment with investigated solutions of filter and free chlorine has lasted for 24 and 48 hours at room temperature, and controlling bulbs were treated with ultra-pure water (zero control). Bulbs underwent the same treatment with control containing free chlorine at concentration of 0.2, 0.4 and 0.6 mg/ml (three controls). Cytogenetic analysis included monitoring the effects of different concentrations of tested UV filter in different concentrations of free chlorine, to the mitotic activity of meristem cells of Allium cepa L., and the types of genotoxic effects expressed during the cell cycle as well. The observed effects were quantitatively presented at the 2000 analyzed cells. Testing the significance of the differences noted between frequencies of each individual and overall aberrations of the meristem cells of onions which were treated with various concentrations of filters at various concentrations of free chlorine, compared to the expected frequencies of the control samples, was performed using Chi-square (χ2) test using the software program for statistical analysis SPSS Statistics 17.0.1.
Results
The first step in the analysis of genotoxicity of homosalate in the presence of the three tested concentrations of free chlorine (0.2, 0.4 and 0.6 mg/ml), using Allium test, was the investigation of the effects of the tested solutions on mitotic activity (mitotic index) of meristem cells of the root of onion, pace of their occurrence and duration, and the frequency of individual mitosis phases. Therefore, investigation included 2000 cells of each of the five bulbs, for each control and each tested concentration.
Mitotic index is expressed in percentage of cell in division in relation to the total number of analyzed cells, and the frequency of each mitotic phase as the absolute number and the percentage of cells in various stages, in relation to the total number of cells in division. The values are evaluated in function of the concentration (10, 30 and 50 ng/ml of tested UV filter in three concentrations of free chlorine: 0.2, 0.4 and 0.6 mg/ml) and the length of treatment (24 and 48 hours).
In the further procedure, microscopic cytogenetic analysis was used to investigate the effect of the solutions on the kinetics, structure and organization of chromosomes in different stages of division of meristem cells of the onion root-Allium cepa L. The results were evaluated on the basis of 200 analyzed mitotic cells, per control and each tested concentration, and are shown as the absolute number and as the percentage of cells with individual and overall aberrations.
Discussion
Allium test genotoxicity investigation was performed on homosalate solutions at concentration of: 10, 30 and 50 ng/ml, and at concentrations of free chlorine: 0.2, 0.4 and 0.6 mg/ml. Chlorinated homosalate by-products occurred in the presence of free chlorine, which was presented in previously published paper [18]. Investigation has shown that the tested solutions, compared to the water as basic control, disrupted mitotic activity of meristem cells of onion root (Table 1). Each test concentration caused a reduction in the mitotic index (MI) compared to the water as a control, after 24 and after 48 hours exposure as well. Statistical analysis demonstrated highly significant differences (p<0.0001) between the MI of water and MI of tested solution. This significant reduction in MI of tested solution compared to MI of water, indicates the high level of inhibition of division that homosalate caused to onion root cells. Along to water as the basic control, the comparison was performed to the three controls represented concentration of free chlorine (0.2, 0.4 and 0.6 mg/ml), which were applied during this test. Majority of the tested concentrations showed highly significant difference in MI compared to MI of their own control containing free chlorine at a specific concentration.
| Treatment length | Concentration of homosalateng/ml | Concentration of free chlorine µg/ml | Analyzed cells | Cells in division | MI % | Prophase | Metaphase | Anaphase | Telophase | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| total | % | total | % | total | % | total | % | ||||||
| 24 h | Control | water | 2000 | 337 | 16.85% | 45 | 13.35% | 139 | 41.25% | 29 | 8.61% | 124 | 36.80% |
| 0.2 | 2000 | 217 | 10.85% | 55 | 25.35% | 88 | 40.55% | 20 | 9.22% | 54 | 24.88% | ||
| 0.4 | 2000 | 172 | 8.60% | 27 | 15.70% | 101 | 58.72% | 16 | 9.30% | 28 | 16.28% | ||
| 0.6 | 2000 | 224 | 11.20% | 39 | 17.41% | 139 | 62.05% | 9 | 4.02% | 37 | 16.52% | ||
| 10 | 0.2 | 2000 | 113 | 5.65% | 33 | 29.20% | 40 | 35.40% | 19 | 16.81% | 21 | 18.58% | |
| 0.4 | 2000 | 121 | 6.05% | 33 | 27.27% | 48 | 39.67% | 14 | 11.57% | 26 | 21.49% | ||
| 0.6 | 2000 | 156 | 7.80% | 33 | 21.15% | 52 | 33.33% | 19 | 12.18% | 52 | 33.33% | ||
| 30 | 0.2 | 2000 | 118 | 5.90% | 22 | 18.64% | 48 | 40.68% | 10 | 8.47% | 38 | 32.20% | |
| 0.4 | 2000 | 143 | 7.15% | 40 | 27.97% | 50 | 34.97% | 8 | 5.59% | 45 | 31.47% | ||
| 0.6 | 2000 | 163 | 8.15% | 45 | 27.61% | 65 | 39.88% | 15 | 9.20% | 38 | 23.31% | ||
| 50 | 0.2 | 2000 | 111 | 5.55% | 14 | 12.61% | 47 | 42.34% | 4 | 3.60% | 46 | 41.44% | |
| 0.4 | 2000 | 150 | 7.50% | 35 | 23.33% | 83 | 55.33% | 2 | 1.33% | 30 | 20.00% | ||
| 0.6 | 2000 | 168 | 8.40% | 12 | 7.14% | 88 | 52.38% | 17 | 10.12% | 51 | 30.36% | ||
| 48 h | Control | water | 2000 | 365 | 18.25% | 60 | 16.44% | 168 | 46.03% | 33 | 9.04% | 104 | 28.49% |
| 0.2 | 2000 | 221 | 11.05% | 51 | 23.08% | 100 | 45.25% | 12 | 5.43% | 58 | 26.24% | ||
| 0.4 | 2000 | 220 | 11.00% | 36 | 16.36% | 92 | 41.82% | 31 | 14.09% | 61 | 27.73% | ||
| 0.6 | 2000 | 237 | 11.85% | 51 | 21.52% | 97 | 40.93% | 26 | 10.97% | 63 | 26.58% | ||
| 10 | 0.2 | 2000 | 143 | 7.15% | 8 | 5.59% | 72 | 50.35% | 20 | 13.99% | 43 | 30.07% | |
| 0.4 | 2000 | 139 | 6.95% | 22 | 15.83% | 68 | 68.00% | 10 | 7.19% | 39 | 28.06% | ||
| 0.6 | 2000 | 172 | 8.60% | 31 | 18.02% | 54 | 31.40% | 24 | 13.95% | 63 | 36.63% | ||
| 30 | 0.2 | 2000 | 150 | 7.50% | 32 | 21.33% | 61 | 40.67% | 12 | 8.00% | 45 | 30.00% | |
| 0.4 | 2000 | 159 | 7.95% | 37 | 23.27% | 59 | 37.11% | 13 | 8.18% | 50 | 31.45% | ||
| 0.6 | 2000 | 168 | 8.40% | 51 | 30.36% | 69 | 41.07% | 12 | 7.14% | 36 | 21.43% | ||
| 50 | 0.2 | 2000 | 120 | 6.00% | 14 | 11.67% | 55 | 45.83% | 10 | 8.33% | 41 | 34.17% | |
| 0.4 | 2000 | 137 | 6.85% | 37 | 27.01% | 47 | 34.31% | 7 | 5.11% | 46 | 33.58% | ||
| 0.6 | 2000 | 154 | 7.70% | 15 | 9.74% | 73 | 47.40% | 7 | 4.55% | 59 | 38.31% | ||
Table 1: Mitotic index (MI) and participation of individual mitotic phases in meristem onion root cells Allium cepa L. after treatment with homosalate solution and free chlorine.
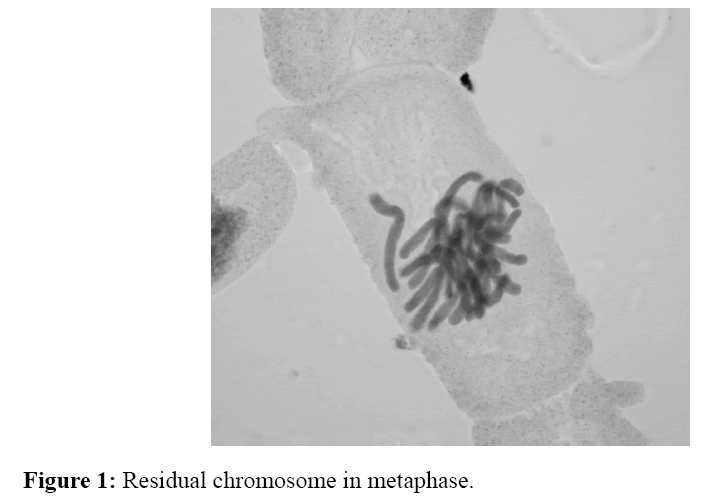
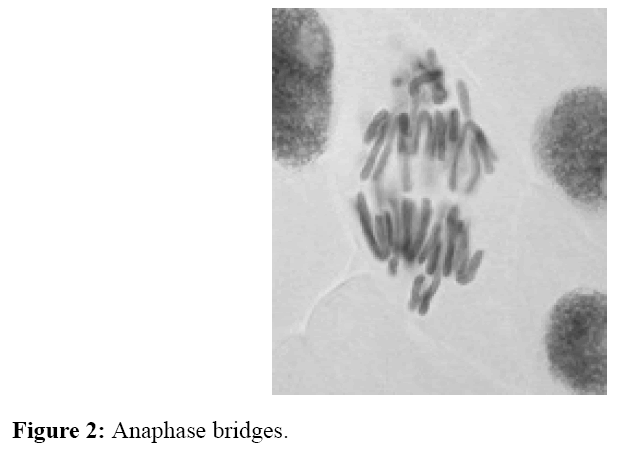
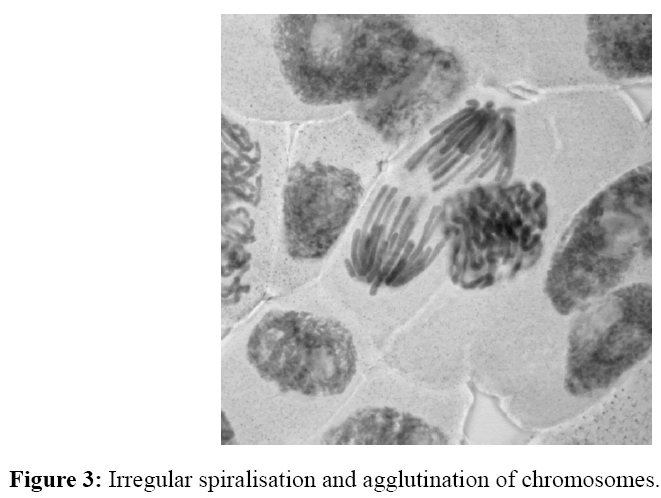
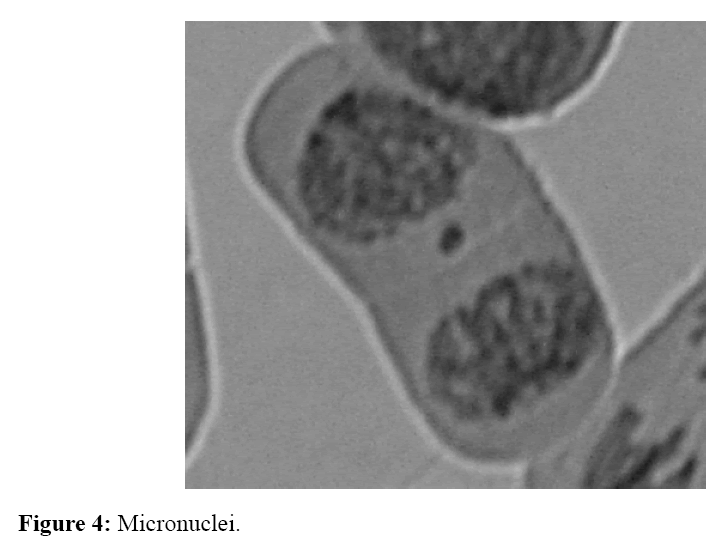
The number of irregular phases caused by tested homosalate solutions was determined by cytogenetic analysis (Table 2). It was found that the tested solutions with applied concentration and at the length of treatment (24 and 48 hours), disturbed kinetics of chromosomes at certain phases of cell division. Aberrations in onion root cells were expressed in the form of disorders of chromosomes kinetic (the highest number) (Figure 1), the appearance of cells with "chromosomal bridge" (Figure 2), cells with agglutinated chromosomes (Figure 3) and micronuclei (Figure 4). Allium test performed on 200 onion root cells, showed statistically significant larger number of aberrations caused by the tested concentrations compared to the water as a control. Most of the tested concentrations showed a statistically significant difference in the number of abnormalities compared to the water as a control. The largest number of aberrations had concentration of 10 ng/ml and 0.2 mg/ml of free chlorine after 24 and 48 hours. The following concentrations showed a significant difference in the number of aberrations, compared to the control containing chlorine: 10 ng/ml - 0.2 mg/ml of free chlorine (0.032 after 24 hours and after 48 hours 0.0036), 0.4 mg/ml of free chlorine (0.009 at 24 hours), 0.6 mg/ml of free chlorine (0.03 after 24 hours); 30 ng/ml-0.2 mg/ml of free chlorine (0.01 after 24 hours), 0,4 mg/ml of free chlorine (0.057 at 24 hours) and 0.6 mg/ml of free chlorine (0.01 after 24 hours), and 50 ng/ml-0.2 mg/ml of free chlorine (0,032 after 24 hours ). It follows from above that the largest number of aberrations in the meristem onion roots cells, were caused by lower concentrations (10 and 30 ng/ml) in the first 24 hours of exposure. From the foregoing, it can be concluded that homosalate by-products occurring in the presence of free chlorine, cause changes at the cellular level by disturbing the kinetics of chromosomes. This results in the appearance of irregular phases and the occurrence of atypical cells. All this leads to the conclusion that homosalate by-products cause genotoxic effects on the onion root cells (Allium cepa L.) and disturb the normal division of meristem cells.
| Treatment length | Concentration of homosalateng/ml | Concentration of free chlorine µg/ml | Cells with disturbed kinetics of chromosomes | Cells with “chromosomal bridge” | Cells with agglutinated chromosomes | „C“ mitosis | Polyploid cells | Cells with aberrations | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| total | % | total | % | total | % | total | % | total | % | total | % | |||
| 24 h | Control | water | 2 | 1.00% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 2 | 1.00% |
| 0.2 | 2 | 1.00% | 0 | 0.00% | 1 | 0.50% | 0 | 0.00% | 0 | 0.00% | 3 | 1.50% | ||
| 0.4 | 5 | 2.50% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 5 | 2.50% | ||
| 0.6 | 3 | 1.50% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 3 | 1.50% | ||
| 10 | 0.2 | 8 | 4.00% | 1 | 0.50% | 2 | 1.00% | 0 | 0.00% | 1 | 0.50% | 12 | 6.00% | |
| 0.4 | 7 | 3.50% | 2 | 1.00% | 2 | 1.00% | 0 | 0.00% | 7 | 3.50% | 18 | 9.00% | ||
| 0.6 | 9 | 4.50% | 1 | 0.50% | 2 | 1.00% | 0 | 0.00% | 0 | 0.00% | 12 | 6.00% | ||
| 30 | 0.2 | 9 | 4.50% | 2 | 1.00% | 2 | 1.00% | 0 | 0.00% | 1 | 0.50% | 14 | 7.00% | |
| 0.4 | 8 | 4.00% | 2 | 1.00% | 4 | 2.00% | 0 | 0.00% | 0 | 0.00% | 14 | 7.00% | ||
| 0.6 | 9 | 4.50% | 3 | 1.50% | 2 | 1.00% | 0 | 0.00% | 0 | 0.00% | 14 | 7.00% | ||
| 50 | 0.2 | 10 | 5.00% | 0 | 0.00% | 1 | 0.50% | 0 | 0.00% | 1 | 0.50% | 12 | 6.00% | |
| 0.4 | 5 | 2.50% | 1 | 0.50% | 2 | 1.00% | 0 | 0.00% | 0 | 0.00% | 8 | 4.00% | ||
| 0.6 | 8 | 4.00% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 8 | 4.00% | ||
| 48 h | Control | water | 2 | 1.00% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 2 | 1.00% |
| 0.2 | 5 | 2.50% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 5 | 2.50% | ||
| 0.4 | 7 | 3.50% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 7 | 3.50% | ||
| 0.6 | 5 | 2.50% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 5 | 2.50% | ||
| 10 | 0.2 | 8 | 4.00% | 3 | 1.50% | 0 | 0.00% | 0 | 0.00% | 4 | 2.00% | 15 | 7.50% | |
| 0.4 | 8 | 4.00% | 4 | 2.00% | 1 | 0.50% | 0 | 0.00% | 0 | 0.00% | 13 | 6.50% | ||
| 0.6 | 6 | 3.00% | 2 | 1.00% | 1 | 0.50% | 1 | 0.50% | 0 | 0.00% | 10 | 5.00% | ||
| 30 | 0.2 | 7 | 3.50% | 1 | 0.50% | 0 | 0.00% | 0 | 0.00% | 1 | 0.50% | 9 | 4.50% | |
| 0.4 | 8 | 4.00% | 2 | 1.00% | 2 | 1.00% | 0 | 0.00% | 0 | 0.00% | 12 | 6.00% | ||
| 0.6 | 10 | 5.00% | 2 | 1.00% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 12 | 6.00% | ||
| 50 | 0.2 | 12 | 6.00% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 12 | 6.00% | |
| 0.4 | 8 | 4.00% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 8 | 4.00% | ||
| 0.6 | 9 | 4.50% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 9 | 4.50% | ||
Table 2: Genotoxic effects of homosalate by-products on meristem onion root cells mitosis.
Conclusion
The aim of this study was to examine the genotoxic effects of homosalate as organic UV filter that is often an ingredient of sunscreens. Also, chlorinated homosalate by-products occur as a result of reaction of homosalate with the free chlorine (swimming pool water). Homosalate and its chlorinated by-products are potential pollutants that reach environment and therefore it was very important to examine their potential genotoxic effects on living organisms.
Results of this study showed that homosalate and its chlorinated by-products occurring in the presence of free chlorine, show genotoxic effects on the onion root (Allium cepa L.) and interfere with the normal division of meristem cells. The results have also provided valuable information in terms of assessing the exposure to UV filters, and the exposure to cosmetic products containing those filters, including their possible effects on living organisms.
References
- Kunz P., Fent K. Estrogenic activity of UV filter mixtures. ToxicolAppl Pharm 2006; 217: 86-99.
- Shaath NA. Ultraviolet filters. PhotochemPhotobiolSci 2010; 9: 464-469.
- Santos AJ., Miranda MS., Esteves da Silva JC. The degradation products of UV filters in aqueous and chlorinated aqueous solutions. Water res 2012; 46: 3167-3176.
- Rodil R., Moeder M. Development of a method for the determination of UV filters in water samples using stir bar sorptive extraction and thermal desorption-gas chromatography-mass spectrometry. J Chromatog A 2008; 1179: 81-88.
- Giokas DL., Salvador A., Chisvert A. UV filters: from sunscreen to human body and the environment. TrAC2007; 26: 360-375.
- Lee Granger K., Brown PR. The chemistry and HPLC analysis of chemical sunscreen filters in sunscreen and cosmetic. JLiqChromatogr R T 2001; 24: 2895-2924.
- Serpone N., Salinaro A., Emeline AV., Horikoshi S., Hidaka H., et al. An in vitro systematic spectroscopic examination of the photostabilities of a random set of commercial sunscreen lotions and their chemical UVB/UVAactive agents. PhotochemPhotobiol S. 2002; 1: 970-981.
- Inbaraj JJ., Bilski P., Chignell CF. Photophysical and photochemical studies of 2-phenylbenzimidazole and UV sunscreen 2-phenylbenzimidazole-5-sulfonic acid. PhotochemPhotobiol 2002; 75: 107-116.
- Zhang S., Chen J., Qiao X., Ge L., Cai X., et al. Quantum chemical investigation and experimental verification on the aquatic photochemistry of the sunscreen 2-phenylbenzimidazole-5-sulfonic acid. Environ SciTechnol 2010; 44: 7484-7490.
- Dı´az-Cruz MS., Llorca M., Barcelo D. Organic UV filters and their photodegradates, metabolites and disinfection by-products in the aquatic environment. TrAC 2008; 27: 873-887.
- Giokas DL., Vlessidis AG. Application of a novel chemometric approach to the determination of aqueous photolysis rates of organic compounds in natural waters. Talanta 2007; 71: 288-295.
- La Farre M., Perez S., Kantiani L., Barcelo D. Fate and toxicity of emerging pollutants, their metabolites and transformation products in the aquatic environment. TrAC 2008; 27: 991-1007.
- Richardson SD., DeMarini DM., Kogevinas M. What’s in the pool? Acomprehensive identification of disinfection by-products and assessment of mutagenicity of chlorinated and brominated swimming pool water. Environ Health Persp 2010; 118: 1523-1530.
- Hrudey SE. Chlorination disinfection by-products, public health risk trade offs and me. Water Res 2009; 43: 2057-2092.
- Monarca S., Feretti D., Zani C., Rizzoni M., Casarella S., et al. Genotoxicity of surface water treated with different disifectants using in situ plant tests. Environ Mol Mutagen 2003; 41: 353-359.
- Nakajima M., Kawakami T., Niino T., Takahashi Y., Onodera S. Aquatic fate of sunscreen agents octyl-4-methoxycinnamate and octyl-4-dimethylaminobenzoate in model swimming pools and the mutagenic assays of their chlorination by-products. J Health Sci 2009; 55: 363-372.
- Vaughn F., Sherwood. Altered UV absorbance and Cytotoxicity of chlorinated sunscreen agents. CutanOculToxicol 2012; 31: 273-279.
- Imamović B., Trifunović S.,Bečić E., Dedić M., Šober M. Study of Homosalatestability in chlorinated water and identification chalogenated by-products by gas chromatography-mass spectrometry. Res J Pharm BiolChemSci 2015; 6: 990-1000.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences